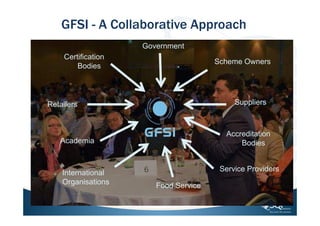
This document provides a summary of a webinar on understanding the top non-conformances in SQF 7.1 certification. It discusses the relationship between SQF and GFSI and the changes between SQF Code editions. The webinar focuses on the most common non-conformances found during SQF certification, re-certification, and surveillance audits for Module 2 (SQF system elements) and Module 11 (food processing). Management responsibility, business continuity planning, food safety plans, internal audits, and corrective action were identified as the most common themes among non-conformances. The webinar provides tips on compliance and ensures understanding of SQF requirements for these areas