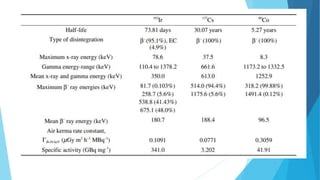
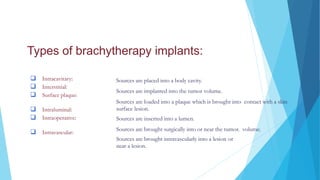
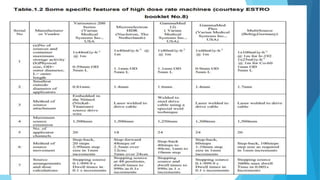
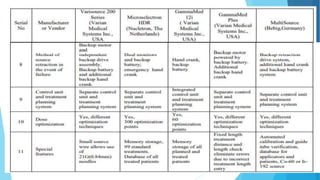
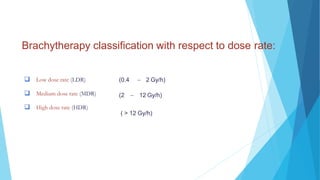
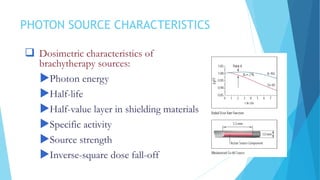
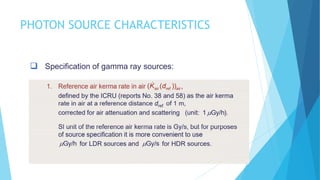
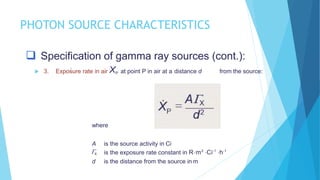
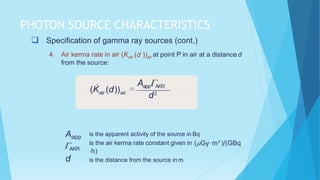
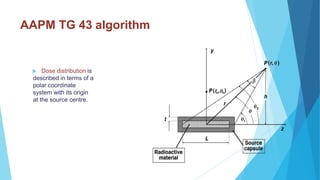
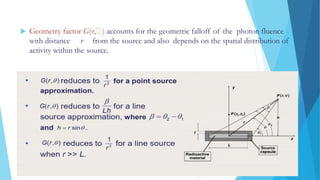
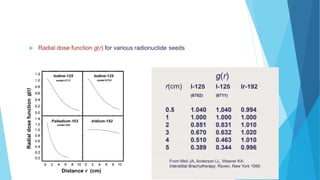
Brachytherapy involves placing radioactive sources inside or near a treatment area. It has advantages over external beam therapy like better localized dose and sharp fall-off outside the target. Common photon sources are cobalt-60, cesium-137, iridium-192, and iodine-125. Dose rate categories are low, medium, and high. Reporting recommendations include describing the technique, dose distribution, and doses to relevant volumes. Proper source specification and calibration are important for accurate treatment.