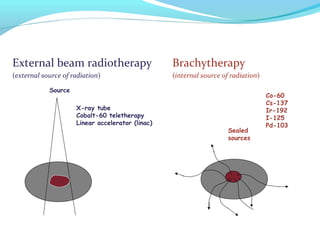
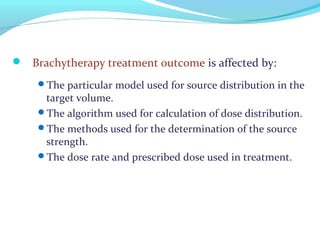
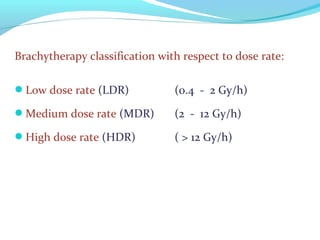
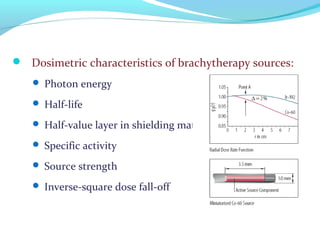
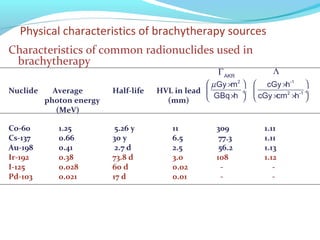
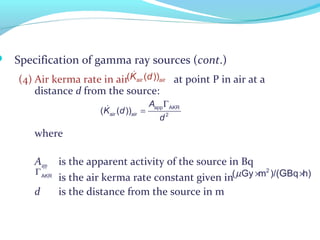
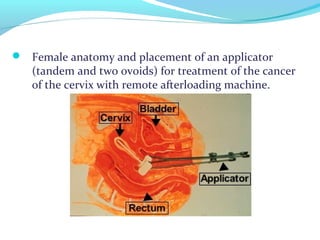
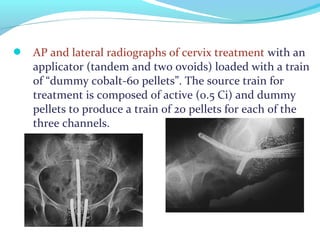
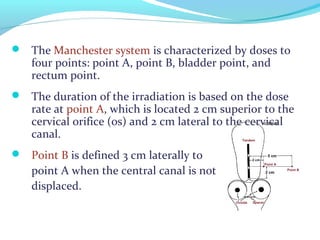
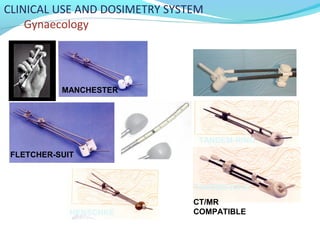
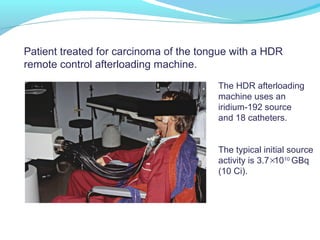
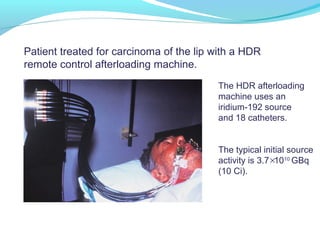
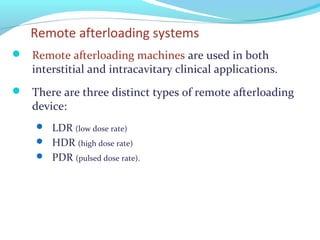
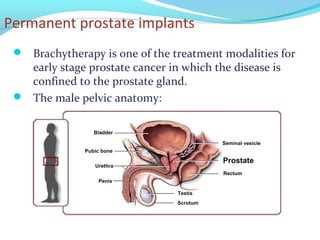
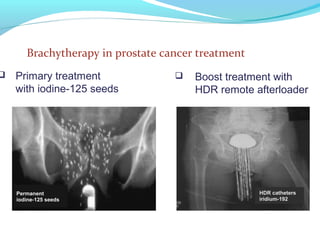
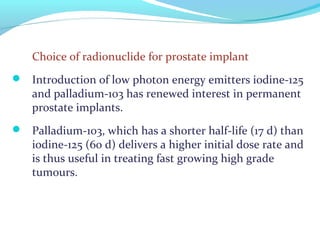
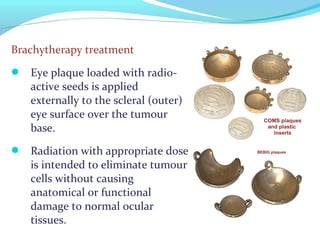
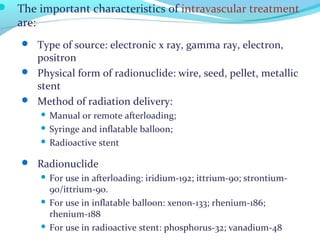
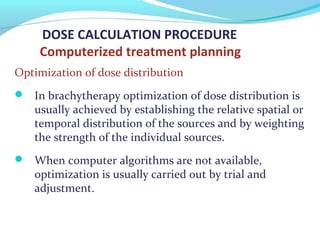
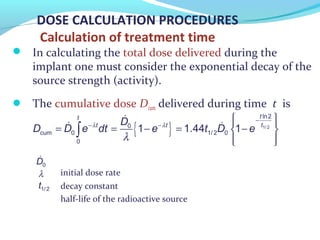
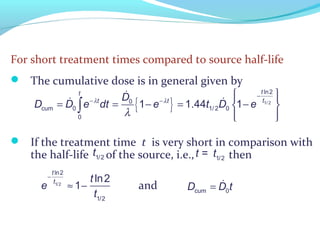
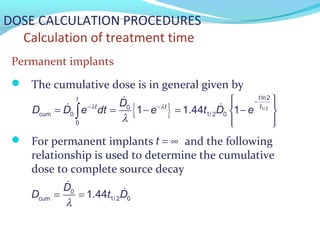
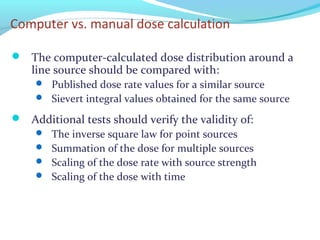
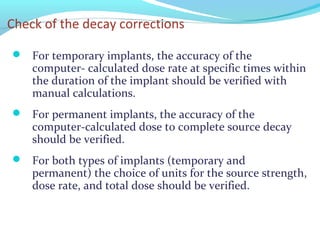
Brachytherapy involves placing radioactive sources inside or near a tumor to deliver radiation. It has advantages over external beam radiation in better targeting the tumor while sparing surrounding healthy tissue. The document discusses the history of brachytherapy and the types of sources, implants, and machines used. It also covers dosimetry systems for gynecological cancers like cervical cancer, which commonly uses intracavitary implants of radioactive sources in an applicator. Interstitial brachytherapy directly implants radioactive sources in the tumor. Remote afterloading machines allow safely implanting and removing radioactive sources.