The document summarizes a novel technique called "bio-milling" that uses fungal biomass to break down chemically synthesized BiMnO3 nanoplates into smaller particles less than 10 nm in size while maintaining crystallinity and phase purity. Specifically:
1) Chemically synthesized BiMnO3 formed nanoplates 150-200 nm in size.
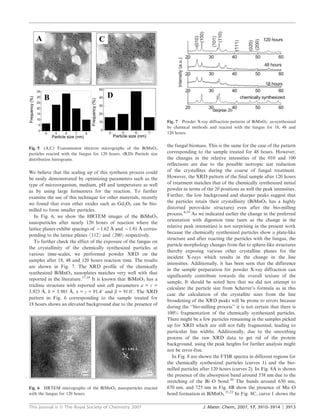
2) Exposure to the fungus Humicola sp. gradually broke the plates down over 120 hours to quasi-spherical particles mostly between 4-8 nm in size.
3) Transmission electron microscopy images show the breakdown process over time and X-ray diffraction confirms the crystalline structure and phase are preserved despite the size reduction.



![We believe that this novel approach of using micro-
organisms in the laboratory environment to break up large
particles into small particles holds tremendous potential in
materials science.
Acknowledgements
The authors P.P. and A.A. would like to acknowledge and
thank the financial support from the Department of Science
and Technology (DST), India to set up a unit on Nanoscience.
One of the authors, P.P., would also like to acknowledge
Fig. 8 FTIR spectra for BiMnO3: (1) as-synthesized by the chemical
a separate grant from SERC, DST, India under the
method, (2) reacted with the fungus.
Nanomission. We acknowledge Mr Gholap, Centre for
Materials Characterization, NCL Pune for assistance with
TEM imaging and Dr P. V. Satyam (Institute of Physics
Bhubneshwar, India) for the HR-TEM imaging.
References
1 C. B. Murray, C. R. Kagan and M. G. Bawendi, Annu. Rev. Mater.
Sci., 2000, 30, 545–610.
2 M. Niederberger, N. Pinna, J. Polleux and M. Antonietti, Angew.
Chem., Int. Ed., 2004, 43, 2270–2273.
3 Y. Sahoo, P. Poddar, H. Srikanth, D. W. Lucey and P. N. Prasad,
J. Phys. Chem. B, 2005, 109, 15221.
4 N. A. Hill, J. Phys. Chem. B, 2000, 104, 6694.
5 X. Li and W. H. Shih, J. Am. Ceram. Soc., 1997, 80, 2844.
6 V. R. Calderone, A. Testino, M. T. Buscaglia, M. Bassoli,
Fig. 9 Preliminary gel electrophoresis (SDS PAGE, pH 8.8) for C. Bottino, M. Viviani, V. Buscaglia and P. Nanni, Chem.
Humicola sp. (HAA-SHC-2) showing four distinct protein bands. Mater., 2006, 18(16), 1627–1633.
7 M. Sastry, A. Ahmad, M. I. Khan and R. Kumar, Curr. Sci., 2003,
85, 2.
absence of amide bands in the chemically synthesized BiMnO3 8 V. Bansal, D. Rautaray, A. Bharde, K. Ahire, A. Sanyal, A. Ahmad
whereas in curve 2 two absorption bands centered around 1658 and M. Sastry, J. Mater. Chem., 2005, 15, 2583.
and 1535 cm21 are attributed to the amide I and II bands 9 V. Bansal, D. Rautaray, A. Ahmad and M. Sastry, J. Mater.
Chem., 2004, 14, 3303.
respectively due to the presence of proteins.23 10 A. Bharde, A. Wani, Y. Shouche, P. A. Joy, B. L. V. Prasad and
To identify the number of proteins secreted by the fungus M. Sastry, J. Am. Chem. Soc., 2005, 127, 9326.
and their molecular weights, the fungus biomass [20.0 g of wet 11 V. Bansal, P. Poddar, A. Ahmad and M. Sastry, J. Am. Chem.
mycelia] was resuspended in 100 mL of sterile distilled water Soc., 2006, 128, 11958–11963.
12 H. L. Ehrlich, Chem. Geol., 1996, 132, 5–9.
for a period of 3 days. The mycelia were then removed by 13 P. Hirsch, F. E. W. Eckhardt and R. J. Palmer, J. Microbiol.
centrifugation, and the aqueous supernatant thus obtained Methods, 1995, 23, 143.
was concentrated by ultra-filtration using a YM3 (molecular 14 V. Bansal, A. Sanyal, D. Rautaray, A. Ahmad and M. Sastry, Adv.
weight cutoff 3000) membrane and then dialyzed thoroughly Mater., 2005, 17, 889.
15 C. N. Mulligan and M. Kamali, J. Chem. Technol. Biotechnol.,
against distilled water using a 3000 cutoff dialysis bag. This 2003, 78, 497.
concentrated aqueous extract containing protein was analyzed 16 J. Y. Son, B. G. Kim, C. H. Kim and J. H. Cho, Appl. Phys. Lett.,
by PAGE (polyacrylamide gel electrophoresis) carried out at 2004, 84, 4971.
pH 8.8.24 In Fig. 9, the preliminary gel electrophoresis 17 V. Samuel, S. C. Navale, A. D. Jadhav, A. B. Gaikwad and
V. Ravi, Mater. Lett., DOI: 10.1016/j.matlet.2006.06.046.
measurement indicates that the fungus secretes four distinct 18 H. Chiba, Solid State Ionics, 1998, 108, 193–199.
proteins ranging in weight from 97 to 20 kDa. One or more 19 Z. H. Chi, C. J. Xiao, S. M. Feng, F. Y. Li and C. Q. Jina, Chem.
of these proteins might be the enzymes that reduce the size Mater., 2005, 17, 1765–1773.
of BiMnO3 and cap BiMnO3 nanoparticles formed by the 20 R. Irmawati, M. N. Noorfarizan Nasriah, Y. H. Taufiq-Yap and
S. B. Abdul Hamid, Catal. Today, 2004, 701, 93–95.
reduction process. It is possible that more than one protein 21 R. A. Nyquist and R. O. Kagel, Infrared spectra of Inorganic
might take part in the capping and stabilization of the BiMnO3 Compounds, Academic Press, New York and London, 1971,
nanoparticles. The exact mechanism leading to the reduction pp. 216–217.
of nanosize BiMnO3 is yet to be elucidated for this fungus. 22 F. A. Alsagheer, M. A. Hasan, L. Pasupulety and M. I. Zaki,
J. Mater. Sci. Lett., 1967, 121, 309.
We are currently separating and concentrating the different 23 D. Rautaray, A. Sanyal, S. D. Adyanthaya, A. Ahmad and
proteins released by the fungus Humicola sp. to test and M. Sastry, Langmuir, 2004, 20, 6827–6833.
identify the ones active in the above processes. 24 U. K. Laemmli, Nature, 1970, 227, 680.
3914 | J. Mater. Chem., 2007, 17, 3910–3914 This journal is ß The Royal Society of Chemistry 2007](https://image.slidesharecdn.com/biomilling-12837702013457-phpapp02/85/Biomilling-5-320.jpg)