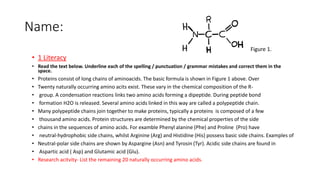
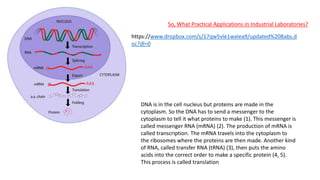
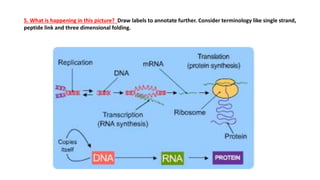
This document provides information about protein synthesis and the role of genes in controlling cellular activities. It discusses how DNA contains instructions in genes that are used to produce mRNA through transcription. The mRNA then directs protein synthesis through translation with the help of tRNA. Proteins fold into complex 3D structures determined by amino acid sequences that allow them to perform specific functions. Issues can arise if errors occur during protein production.