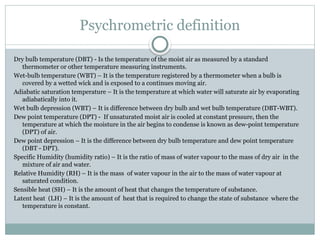
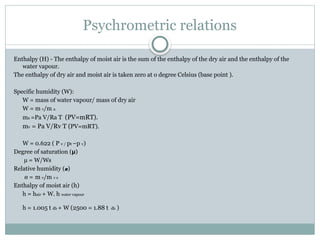
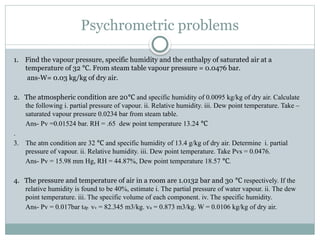
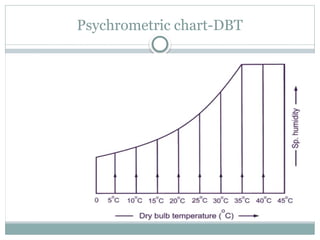
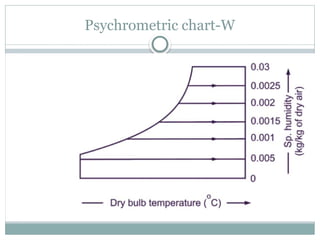
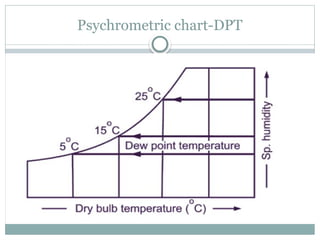
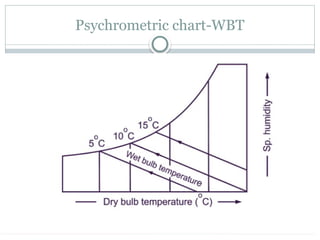
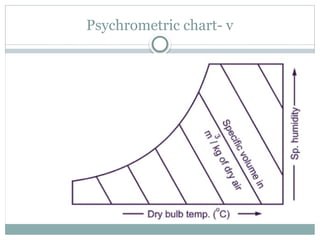
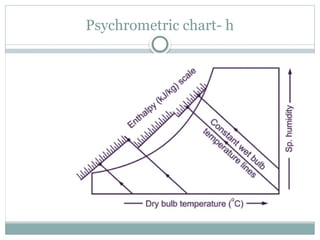
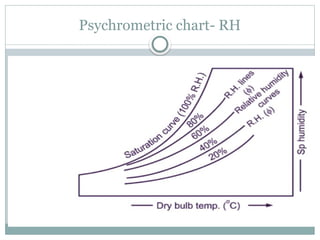
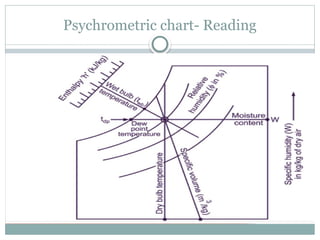
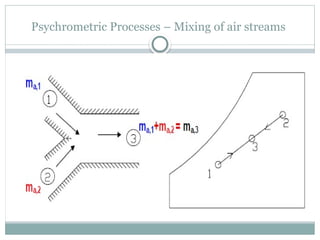
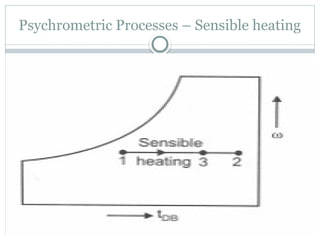
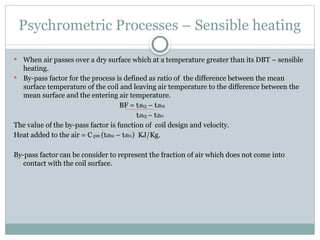
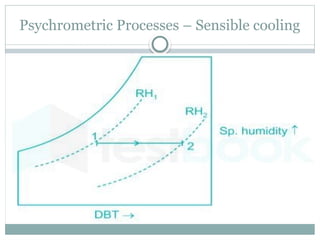
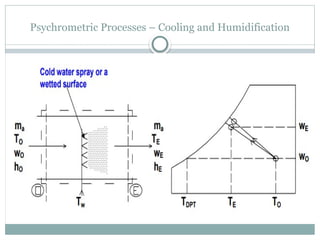
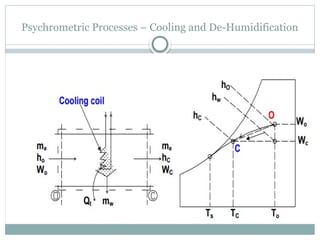
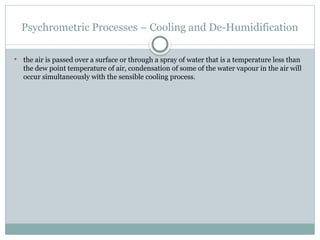
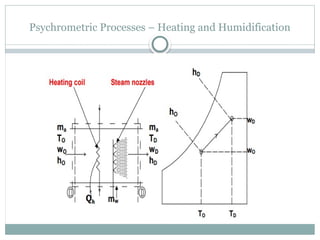
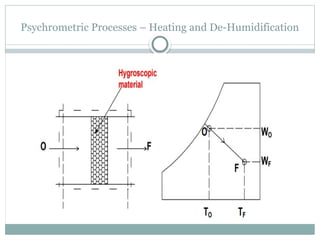
The document discusses psychrometry, focusing on the properties of moist air, which is a mixture of dry air and water vapor. It covers key concepts such as dry bulb temperature, wet bulb temperature, specific humidity, relative humidity, and various psychrometric processes like heating, cooling, and humidification. Additionally, it includes practical problems related to calculating vapor pressure, relative humidity, and dew point temperature based on given conditions.