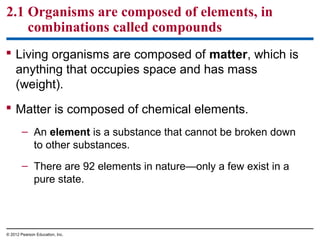
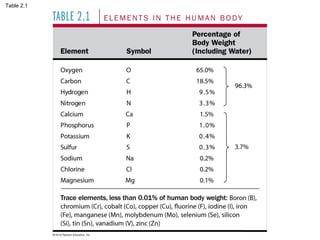
- Living organisms are composed of chemical elements like carbon, hydrogen, oxygen, and nitrogen, which combine to form compounds. Compounds are made of two or more elements bonded together.
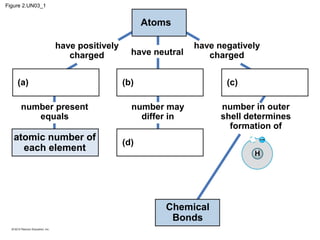
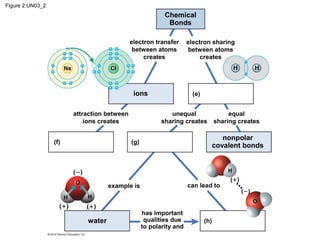
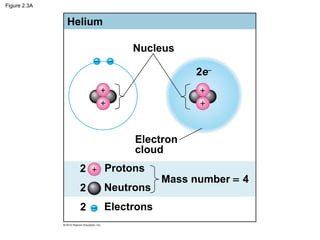
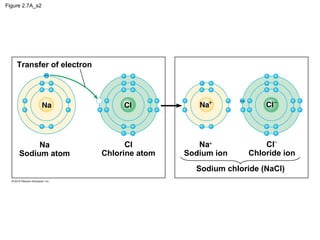
- Atoms are the basic units that make up elements. Atoms contain protons, neutrons, and electrons. Chemical bonds like covalent bonds and ionic bonds form when atoms interact and share or transfer electrons.
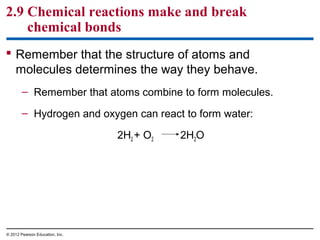
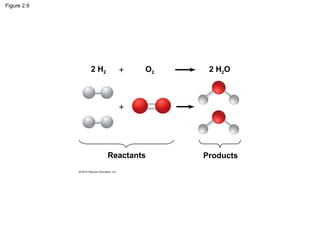
- Different types of chemical bonds like covalent bonds, ionic bonds, and hydrogen bonds are important for forming molecules and determining molecular structures and properties. Chemical reactions make and break these bonds by rearranging atoms.












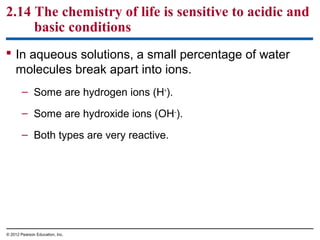
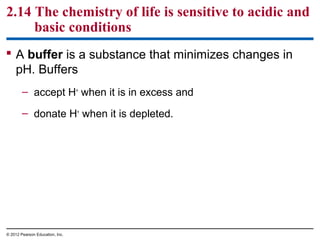
![Figure 2.14_1
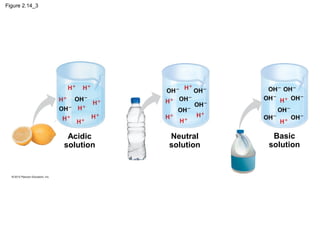
IncreasinglyACIDIC
(HigherH+
concentration)
Tomato juice
pH scale
Battery acid
Lemon juice,
gastric juice
Vinegar, cola
NEUTRAL
[H+
]=[OH−
]
Rainwater
Human urine
Saliva
Pure water](https://image.slidesharecdn.com/bioch2-151024194935-lva1-app6891/85/Bio-ch2-49-320.jpg)
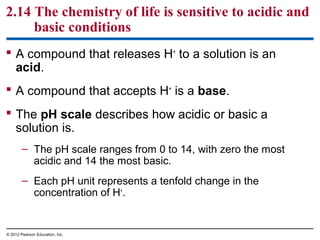
![Figure 2.14_2
Oven cleaner
IncreasinglyBASIC
(HigherOH−
concentration)
NEUTRAL
[H+
]=[OH−
]
Household bleach
Household ammonia
Milk of magnesia
Pure water
Human blood,
tears
Seawater
pH scale](https://image.slidesharecdn.com/bioch2-151024194935-lva1-app6891/85/Bio-ch2-50-320.jpg)