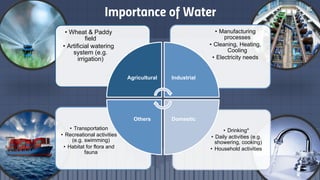
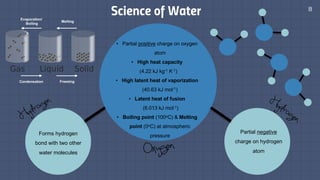
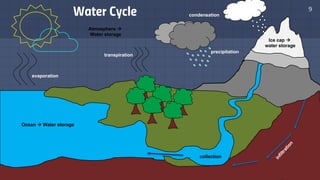
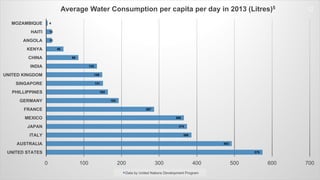
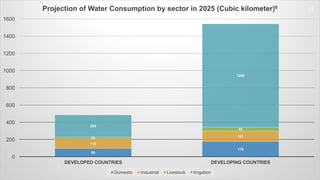
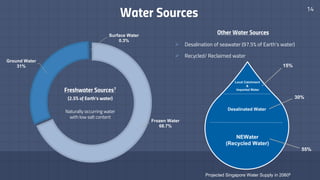
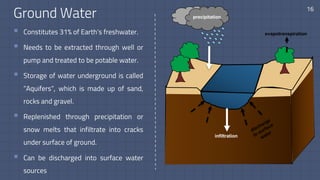
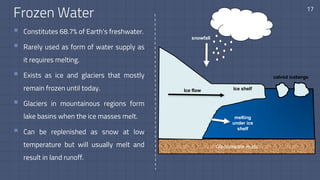
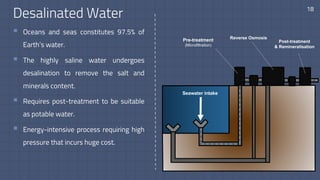
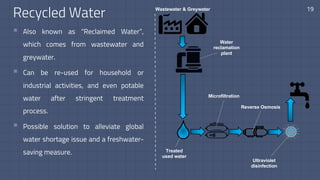
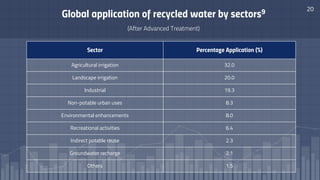
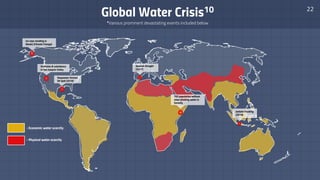
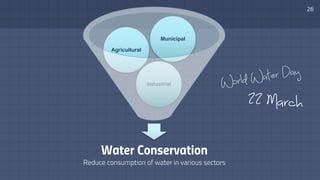
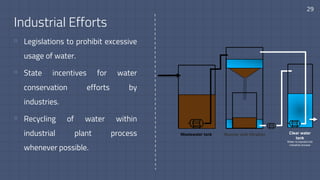
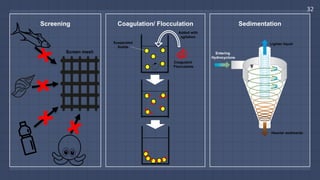
The document discusses the essential role of water in sustaining life and outlines its various functions, sources, and the global water crisis. It details water management practices, conservation strategies, and purification methods to address water scarcity. Additionally, it presents statistics related to global water consumption and highlights the importance of sustainable water resource management.















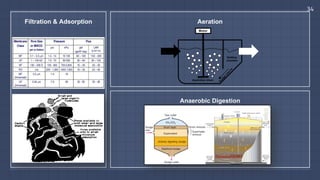
![Filtration & Adsorption
▫ Filtration is the physical or mechanical
process of separating solids from
liquids through a membrane.
▫ [Pore sizes of Ultrafiltration: 0.002 –
0.1 microns, Nanofiltration: 0.001
microns, Reverse Osmosis: Removes
everything]
▫ Carbon filtering uses a bed of
activated carbon to remove
contaminants through chemical
adsorption. It is used to remove
organic compounds and free chlorine,
preventing discharge of harmful
byproducts.
33
Water Purification – Stage 2
Filtration
Aeration
▫ Aeration is a process of introducing
air into the water through bubbling.
▫ This is done to reduce the unpleasant
smell and taste by inducing oxidation
of dissolved or suspended metals and
volatile organic chemicals (VOCs).
▫ Dissolved gases such as carbon
dioxide can also be removed through
aeration.
Anaerobic Digestion
▫ Anaerobic Digestion is the introduction
of microorganisms such as bacteria
into the water in order to break down
biodegradable materials.
▫ This process is done with little or no
presence of oxygen, with high
application in wastewater treatment.
▫ It can effectively eliminate pathogens
as well as lowering the Chemical
Oxygen Demand (COD) in the
wastewater.](https://image.slidesharecdn.com/waterslides-180614011403/85/Basics-of-Water-Purification-Methods-33-320.jpg)