This document provides an overview of basic principles of liver resection, including:
- A brief history of liver resection and techniques like hepatic inflow occlusion.
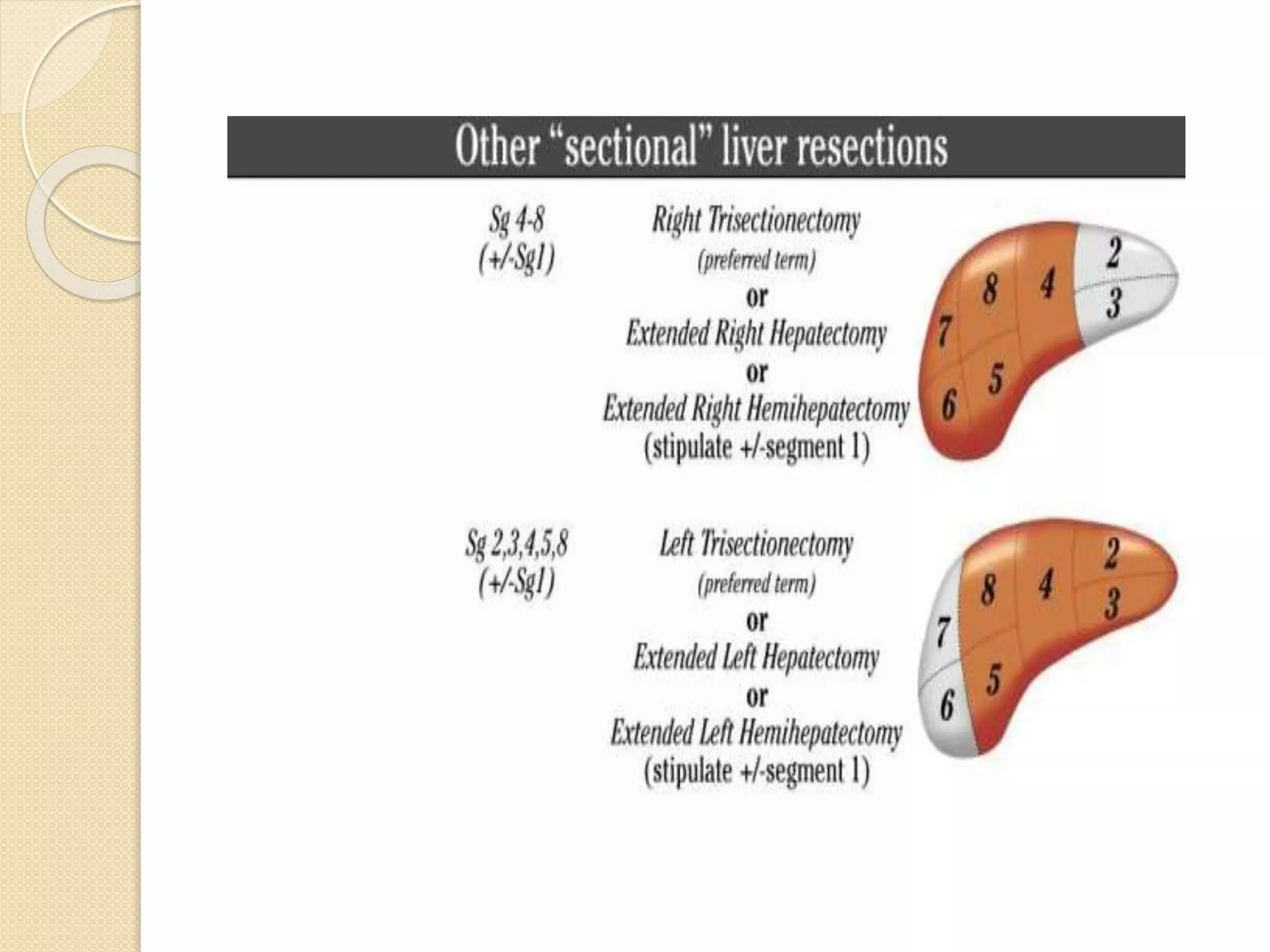
- Concepts of liver regeneration, surgical anatomy, and terminology as described by Couinaud.
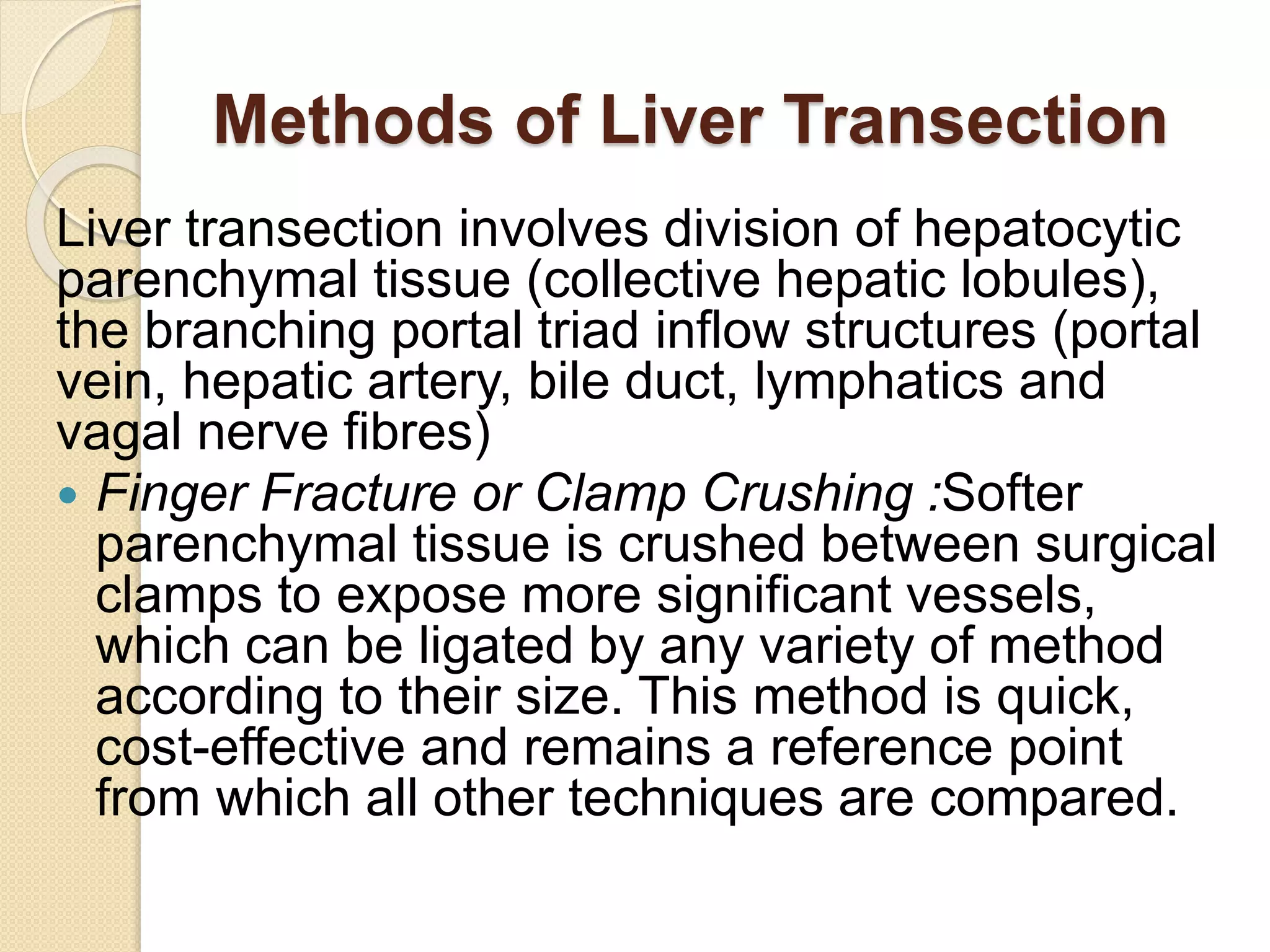
- Surgical techniques for liver transection including finger fracture, water jet, CUSA, Ligasure, and vascular staplers.
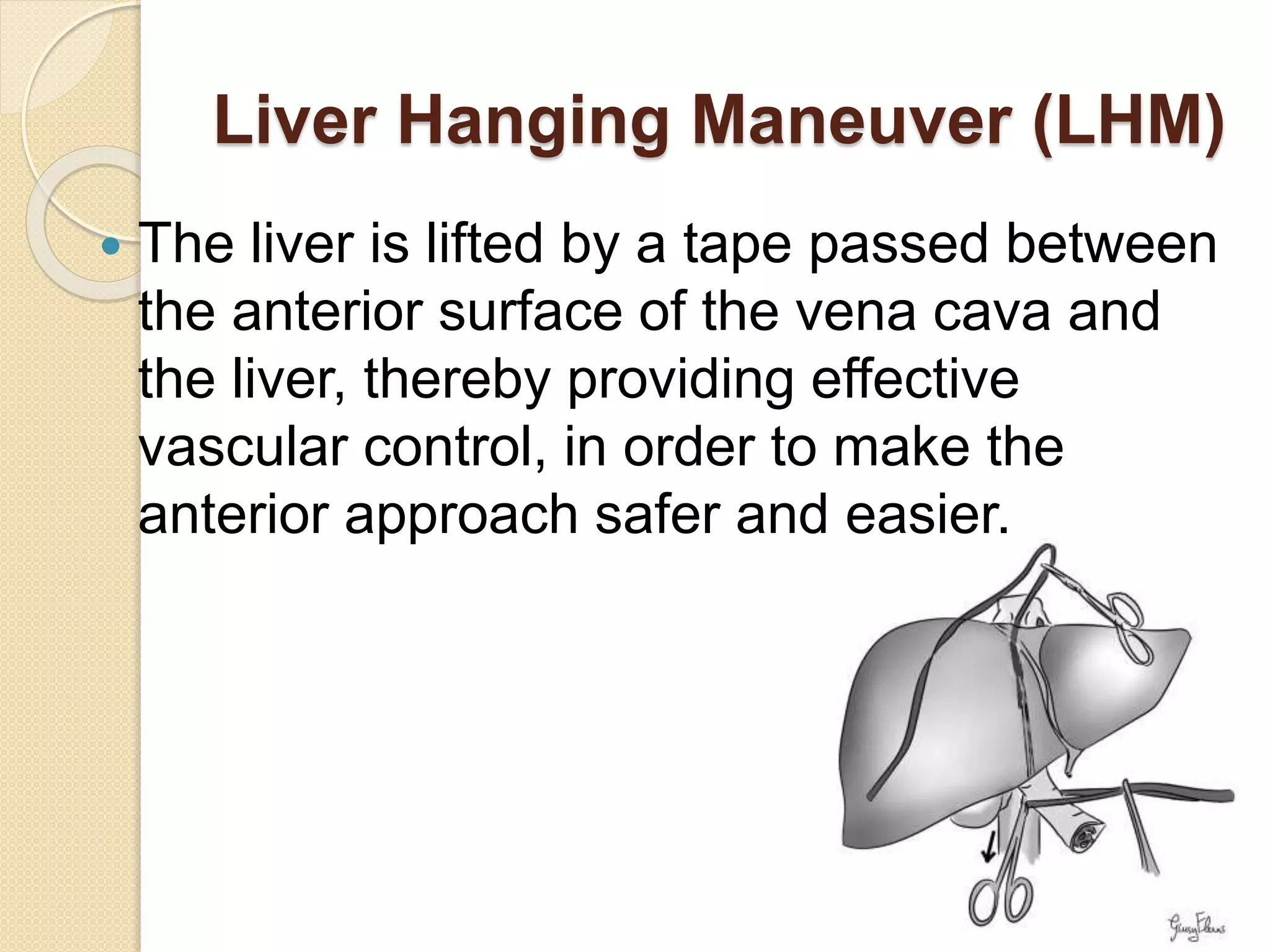
- Methods of vascular control during resection like Pringle maneuver, liver hanging maneuver, and total hepatic vascular exclusion.
- Postoperative management considerations like fluid/electrolyte balance, nutrition, pain control, and monitoring for liver failure.