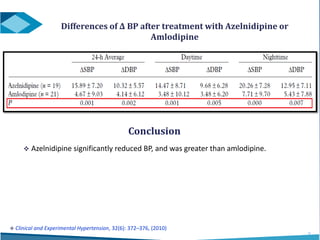
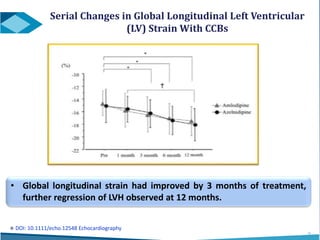
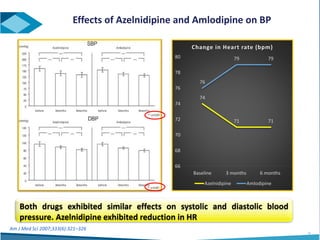
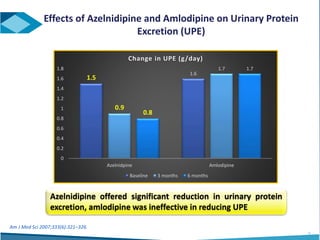
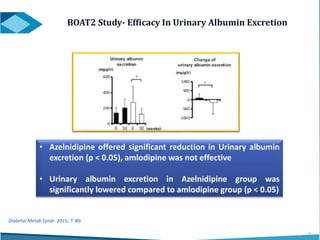
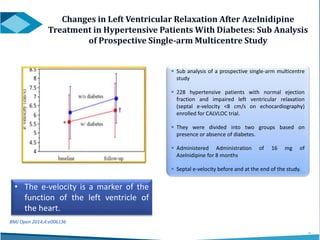
Azelnidipine is a novel dihydropyridine calcium channel blocker (CCB) effective in treating essential hypertension, exhibiting superior lipophilicity and combined L- and T-type channel-blocking mechanisms. Clinical studies indicate that it provides sustained blood pressure control, reduces proteinuria, and has fewer side effects such as reflex tachycardia compared to traditional CCBs like amlodipine. Azelnidipine is distinguished by its renoprotective effects and potential benefits in managing hypertension in populations susceptible to cardiovascular events.













![Study 1: Sustained Blood Pressure-Lowering Effect of Azelnidipine
Guided by Self-Measured Morning and Evening Home Blood
Pressure: Subgroup Analysis of the At-HOME Study
• At-HOME study conducted in Japan
• Objective: To evaluate the sustained BP lowering effect of Azelnidipine,
using mean morning and evening systolic BP [ME average] and morning
systolic BP minus evening systolic BP (ME difference).
• Efficacy analysis- N = 4852
• Safety analysis- N = 5265
18
Drugs R D (2013) 13:75–85](https://image.slidesharecdn.com/azelnidipine-200611104836/85/Azelnidipine-18-320.jpg)

![Improvement in Patient Distribution
Drugs R D (2013) 13:75–85
20
Changes in patient distribution according to morning and evening systolic blood pressure (ME average) and morning systolic blood
pressure minus evening systolic blood pressure (ME difference) [n = 2,101; p0.0001 vs. baseline according to the McNemar test].
• Significant reduction in home SBP and DBP
• BP-lowering effect lasted till next day morning
• Useful for patients with morning hypertension, who are at high risk
of cardiovascular events, especially stroke.](https://image.slidesharecdn.com/azelnidipine-200611104836/85/Azelnidipine-20-320.jpg)