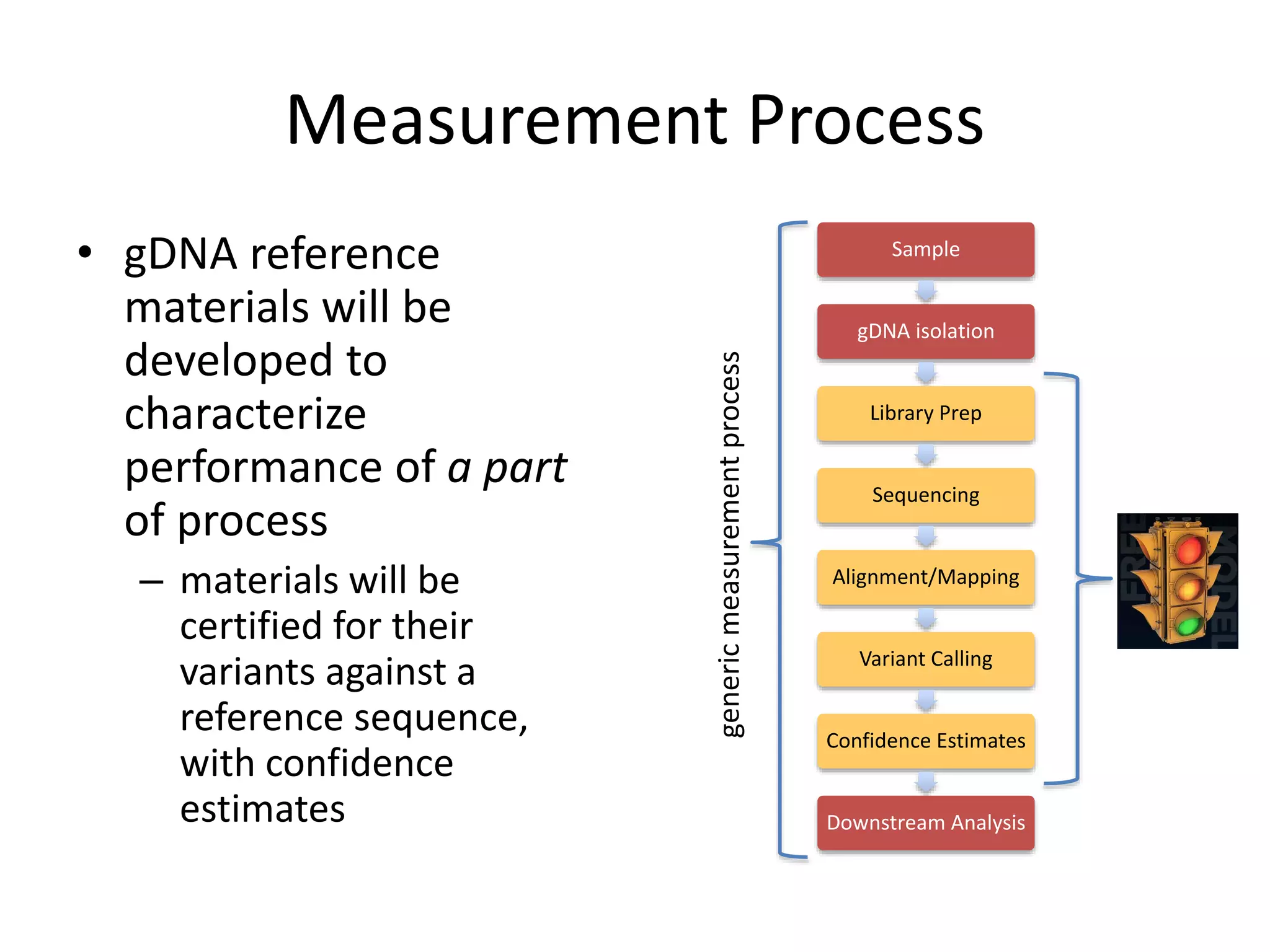
The Genome in a Bottle Consortium held a workshop in August 2014 at NIST to discuss progress and plans.
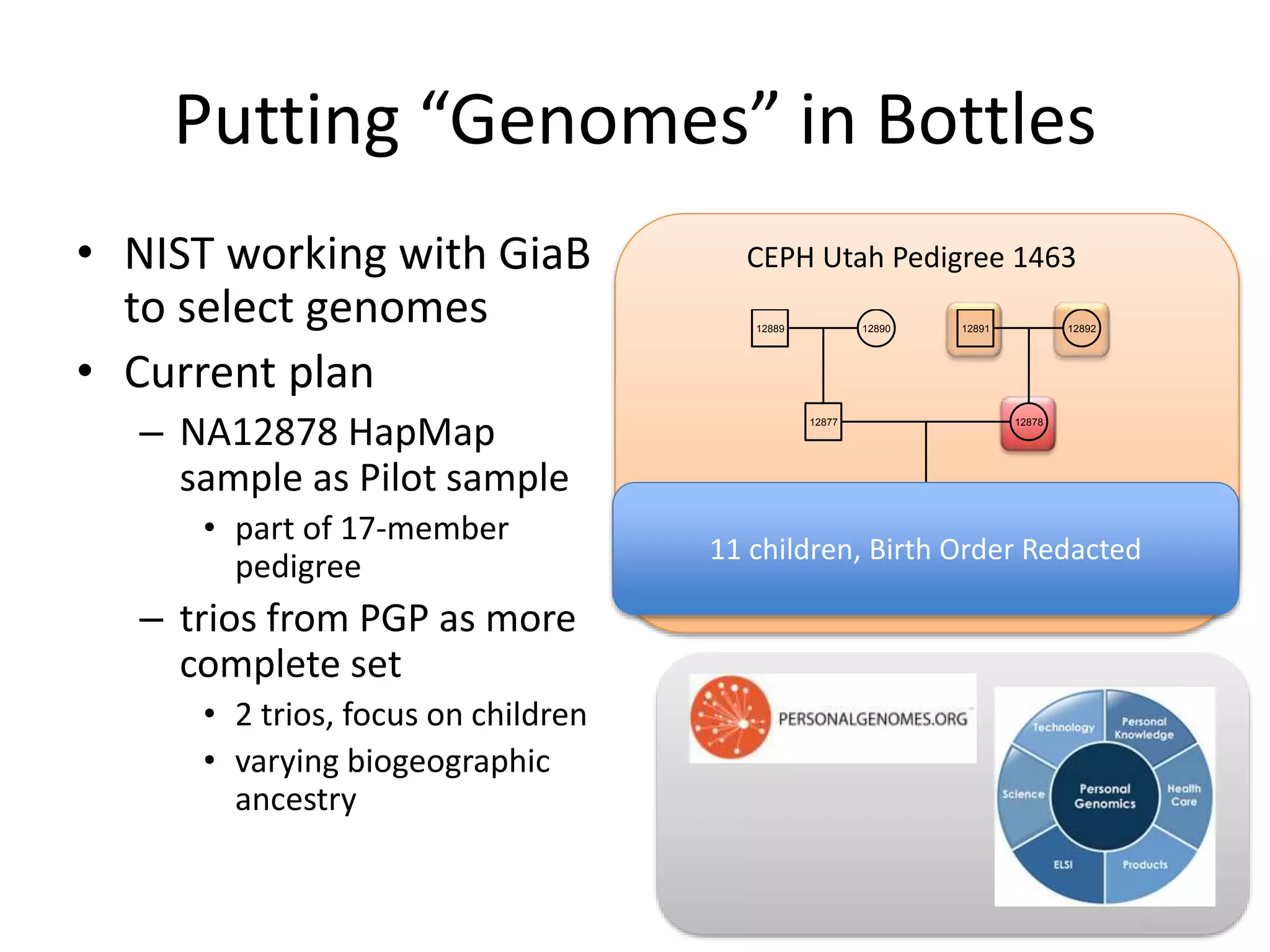
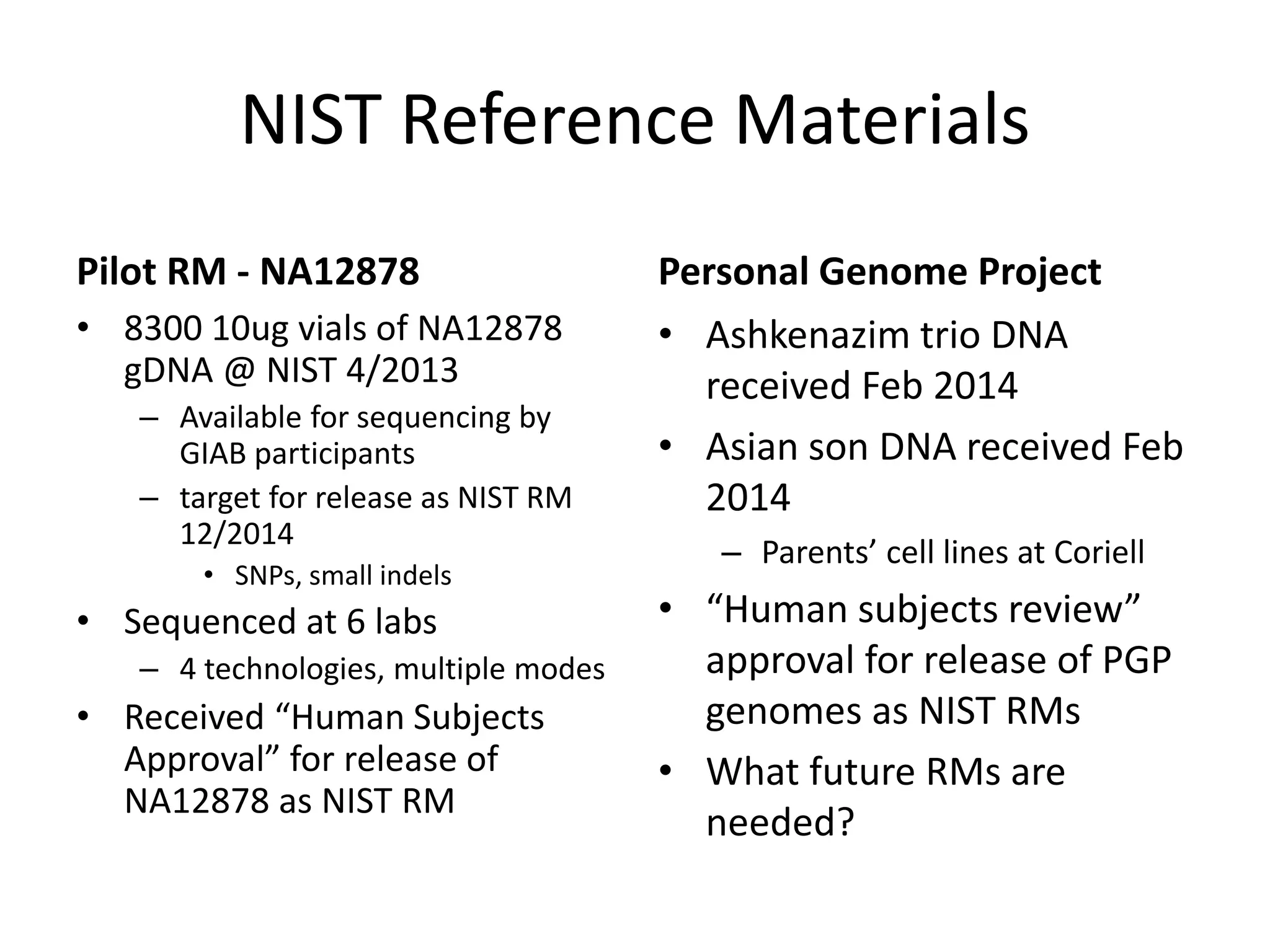
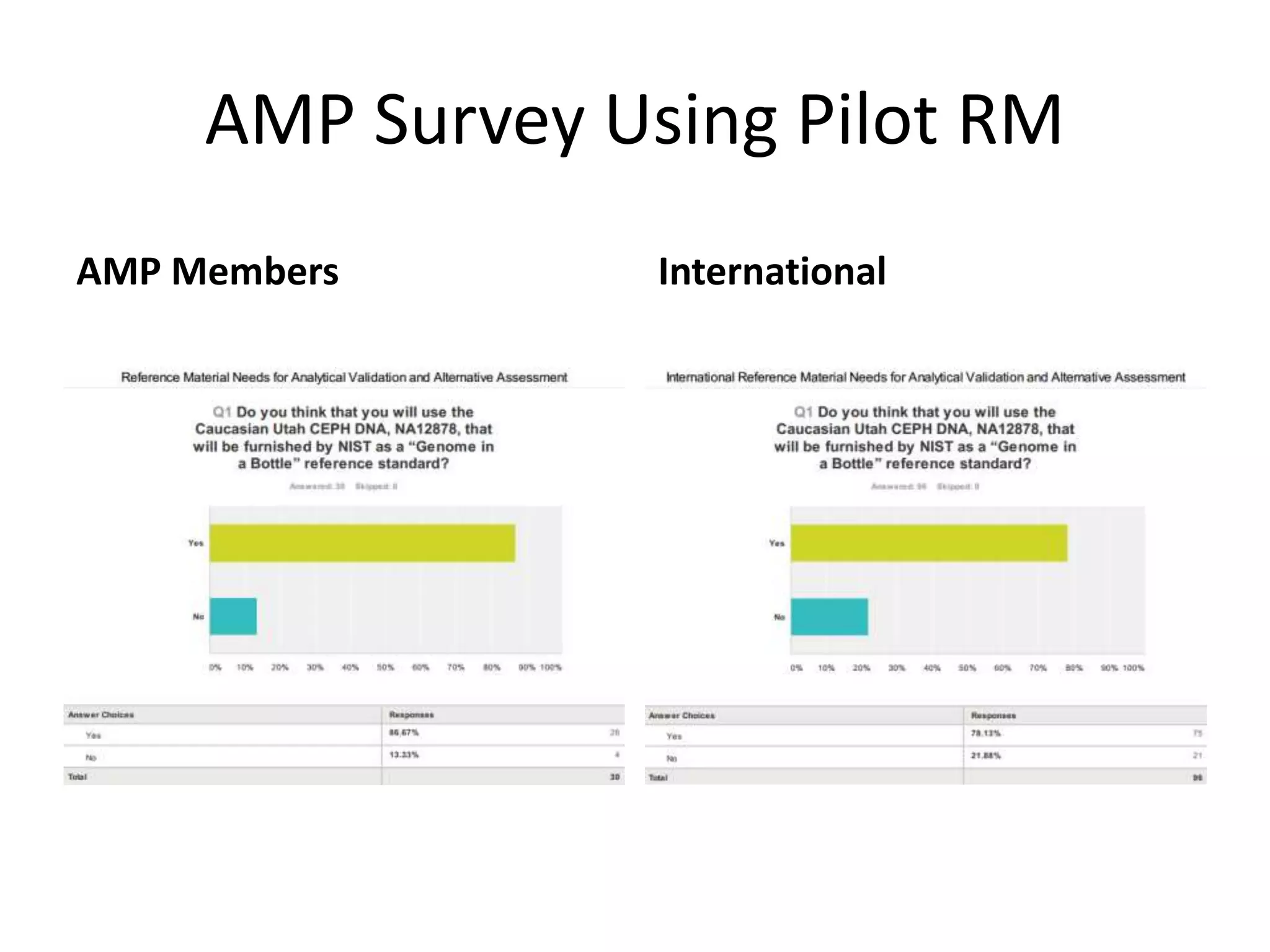
- They have developed a pilot reference material (RM) using the genome of individual NA12878, releasing 8,300 vials to participants for sequencing and analysis.
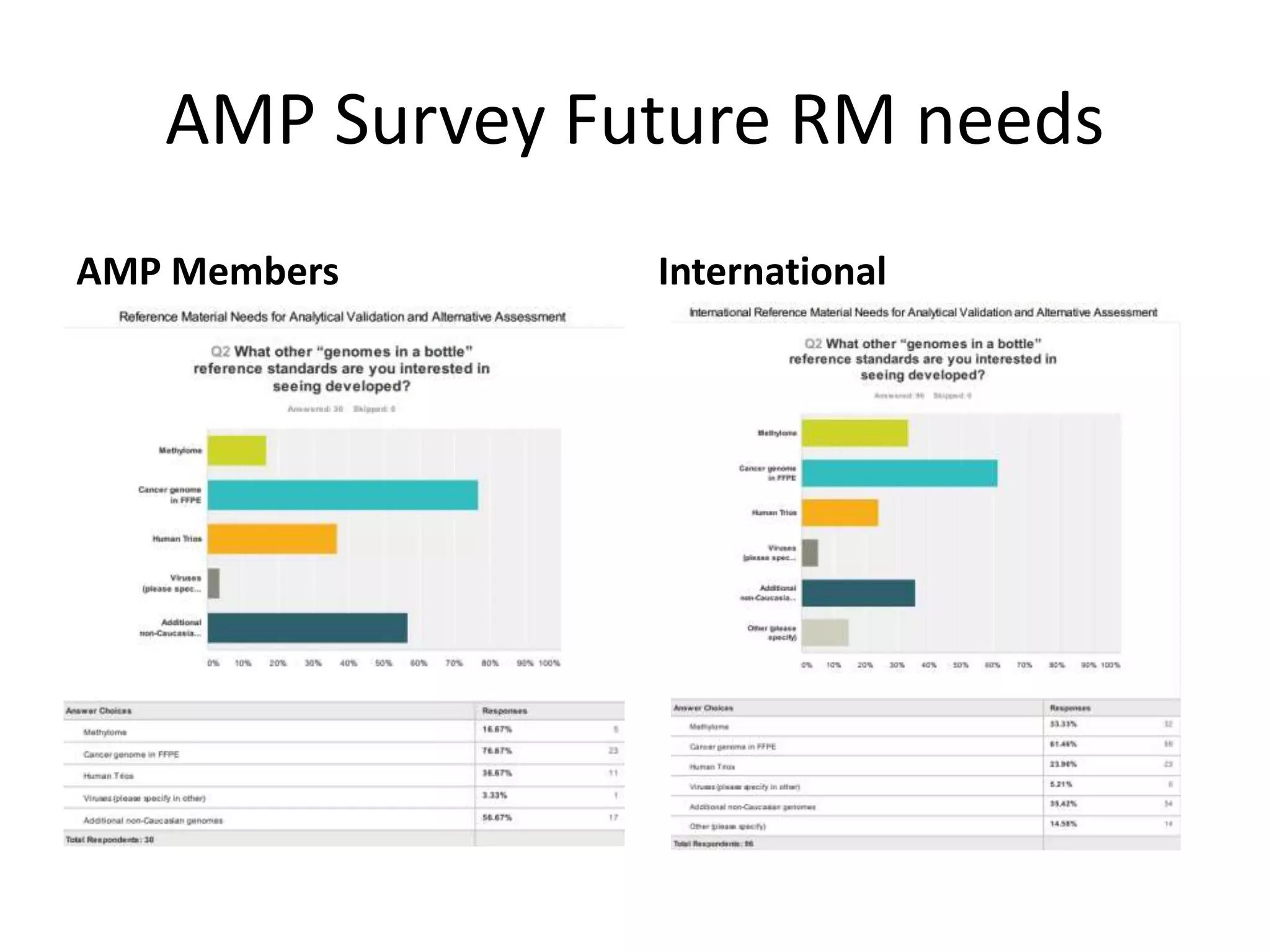
- They are working to characterize additional genomes from the Personal Genome Project, including an Ashkenazi Jewish trio and their Asian son, to serve as future reference materials.
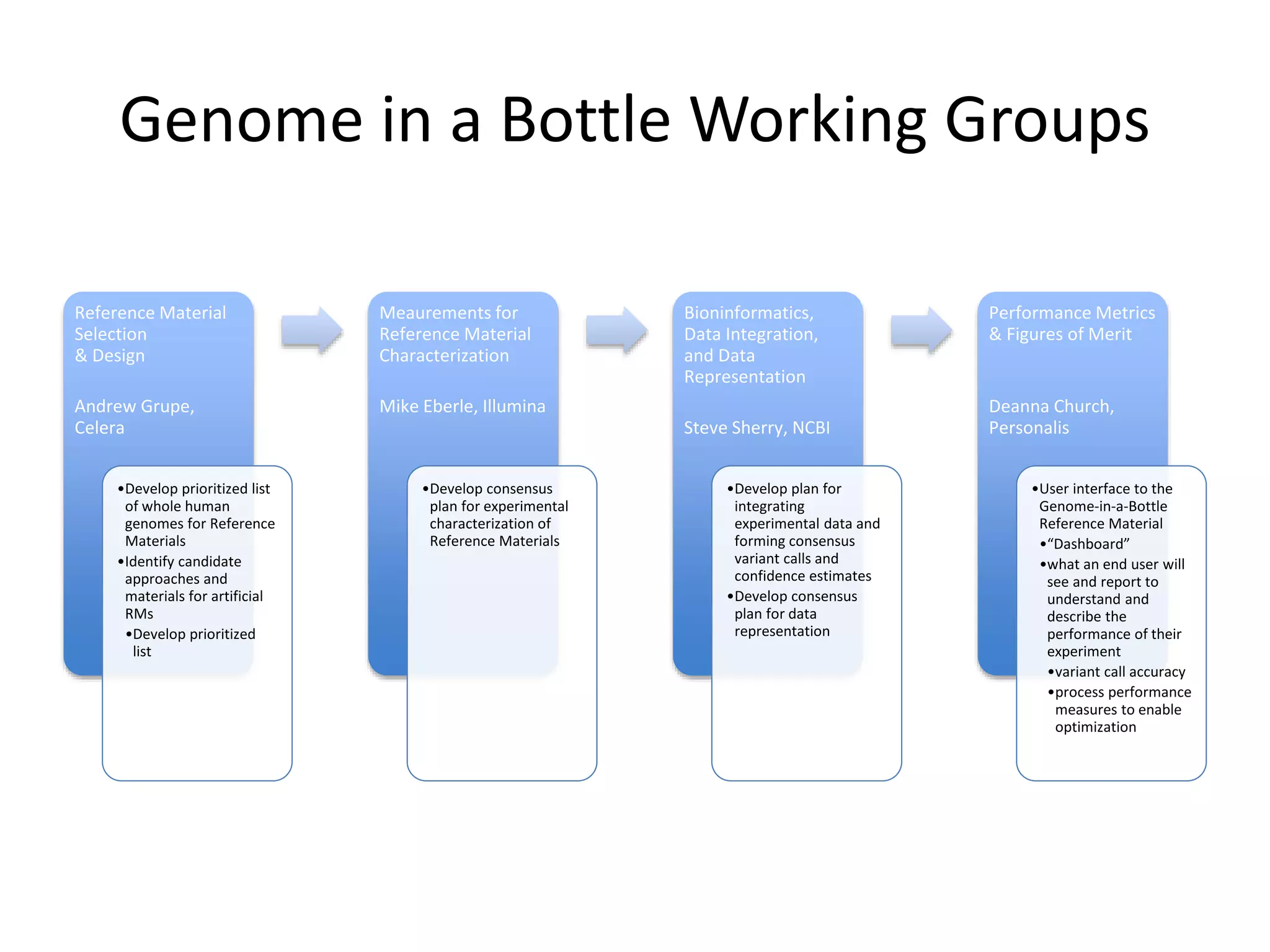
- At the workshop they discussed expanding the types of variants characterized in reference materials, selecting future genomes, and obtaining necessary approvals to release human genomes as reference materials.