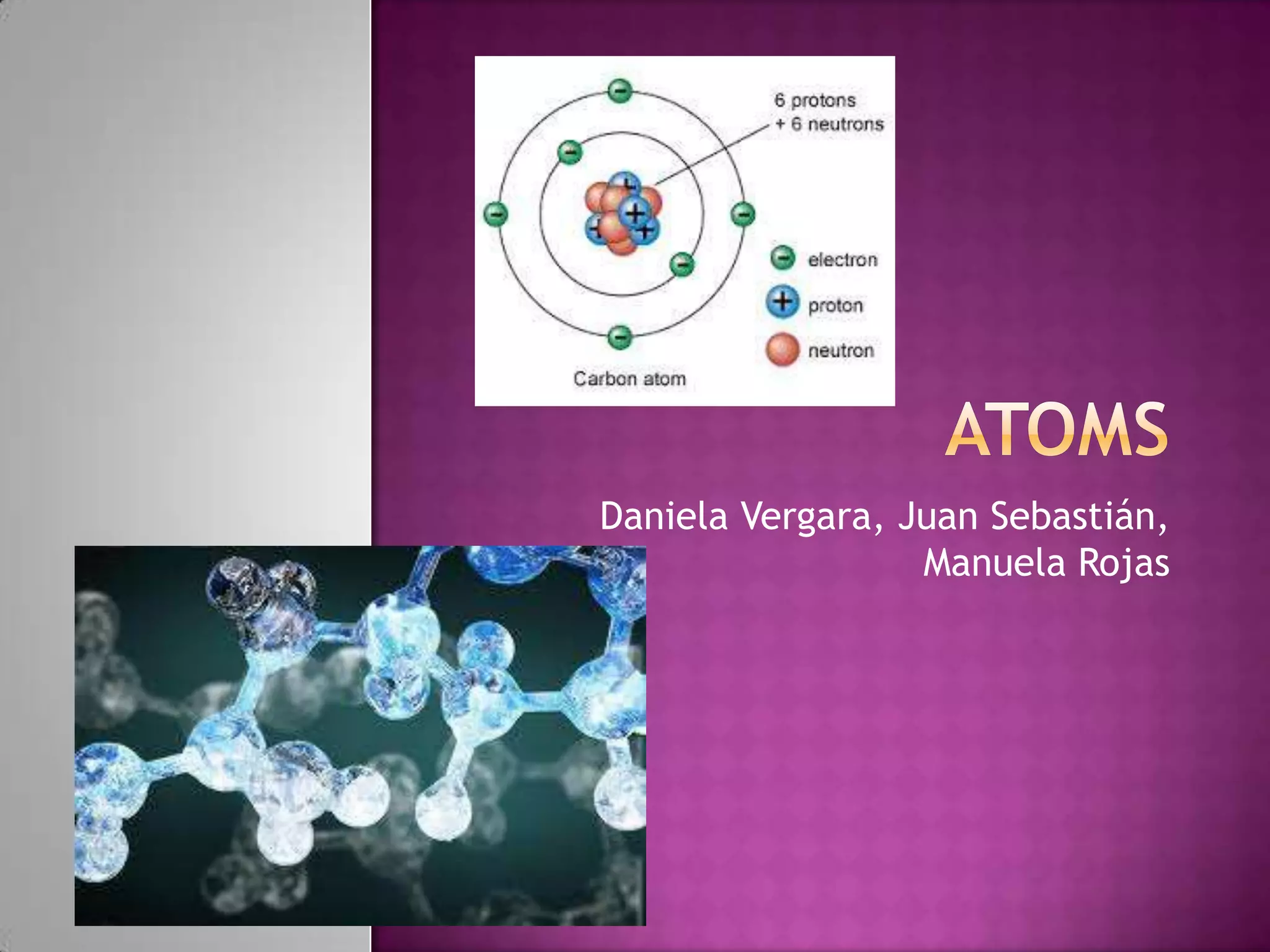
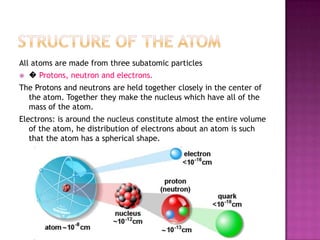
1) Atoms are composed of protons, neutrons, and electrons. Protons and neutrons are clustered in the nucleus, while electrons surround the nucleus and give the atom its shape.
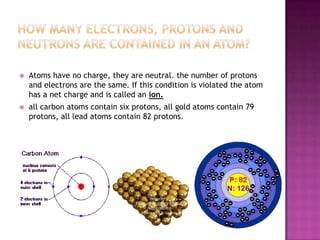
2) Atoms are neutral when they have an equal number of protons and electrons. Ions are formed when there is an imbalance of protons and electrons.
3) Atoms of the same element all contain the same number of protons. For example, all carbon atoms have 6 protons. Atoms can fuse or split to form heavier or lighter atoms through nuclear reactions.