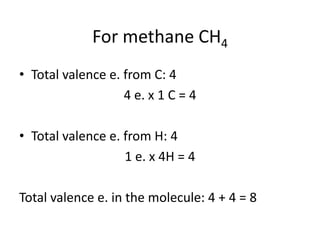
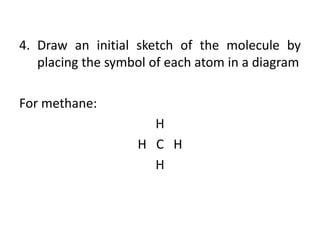
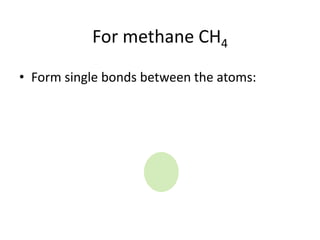
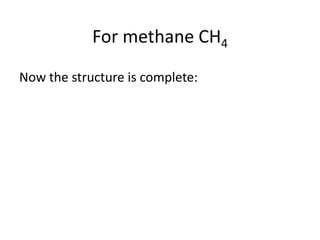
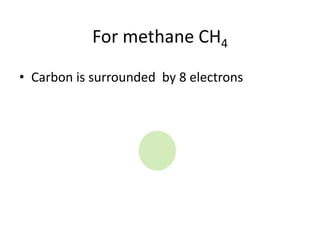
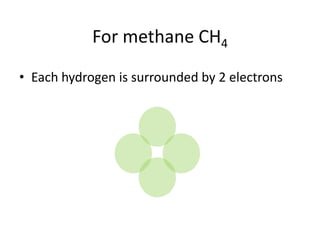
The document provides instructions for drawing the Lewis structure of methane (CH4). It explains that you first determine the number of valence electrons for each atom, which is usually the same as the group number on the periodic table. For methane, carbon contributes 4 electrons and each hydrogen contributes 1. These are summed to get the total of 8 valence electrons. Bonds are then drawn between the atoms to give each atom an octet of electrons, following the octet rule. The completed Lewis structure for methane shows each carbon bonded to 4 hydrogens and each hydrogen bonded to the carbon.