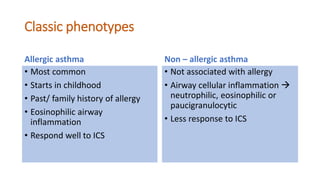
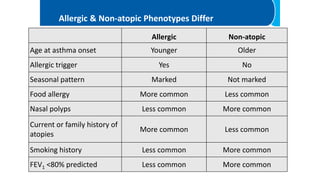
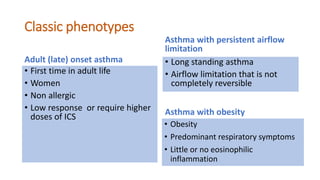
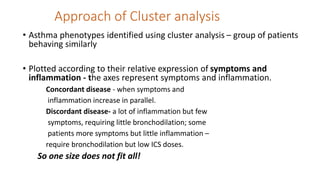
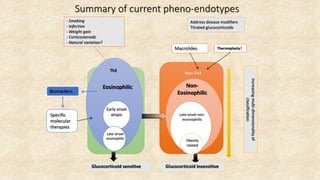
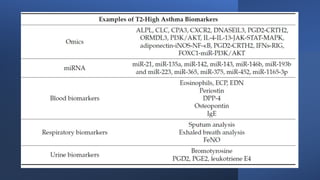
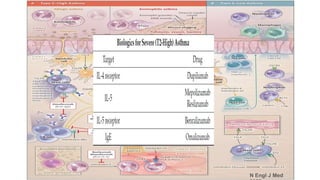
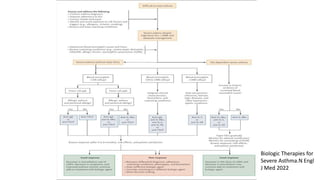
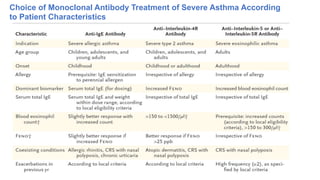
Asthma is a heterogeneous disease characterized by diverse phenotypes influenced by clinical, demographic, and pathophysiological factors, with implications for treatment responsiveness. Phenotyping aids in understanding the disease's aetiology and developing targeted therapeutic approaches, particularly in severe cases. The document emphasizes the transition from phenotypes to endotypes to personalize asthma treatment based on specific biological mechanisms.