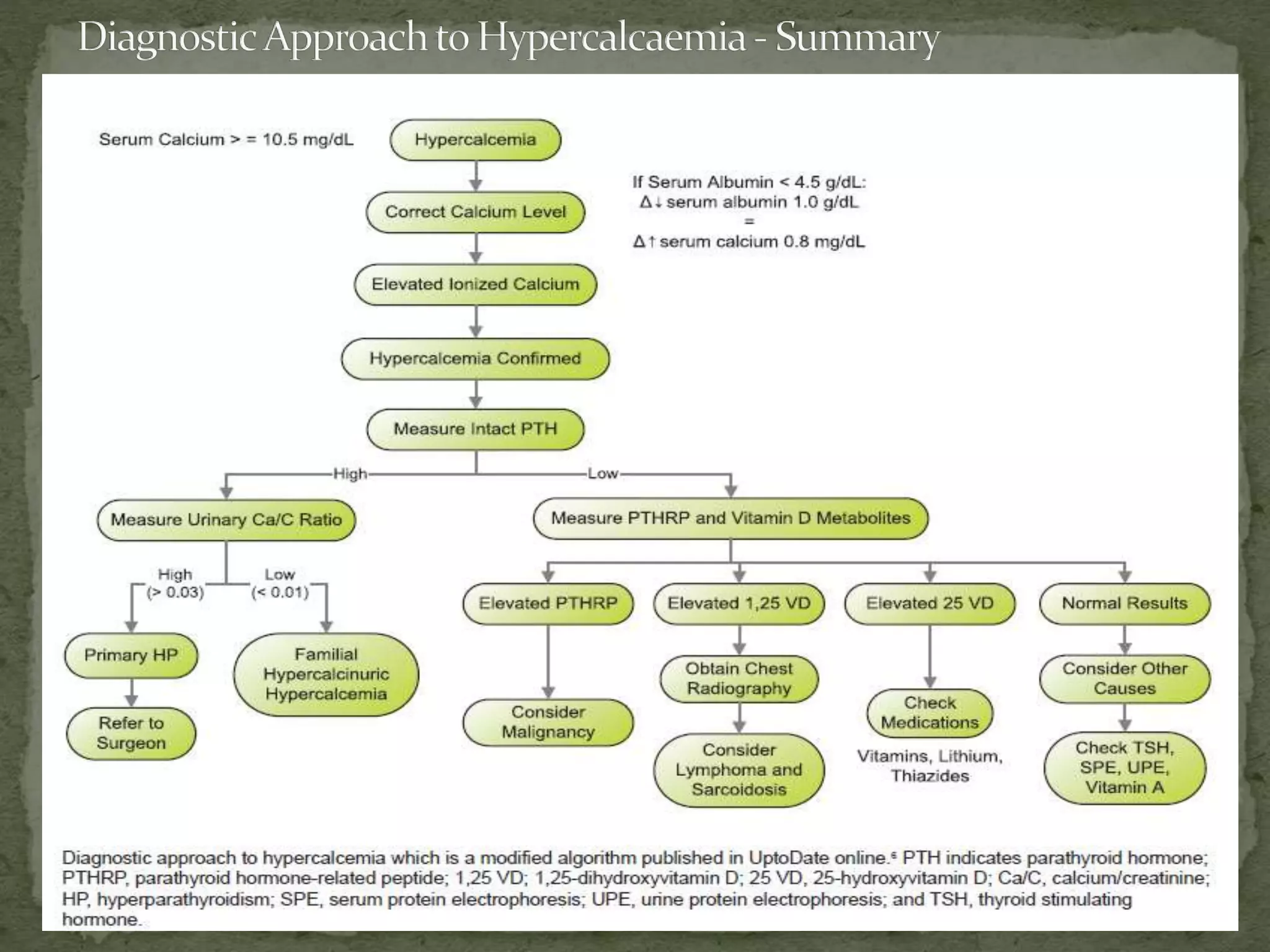
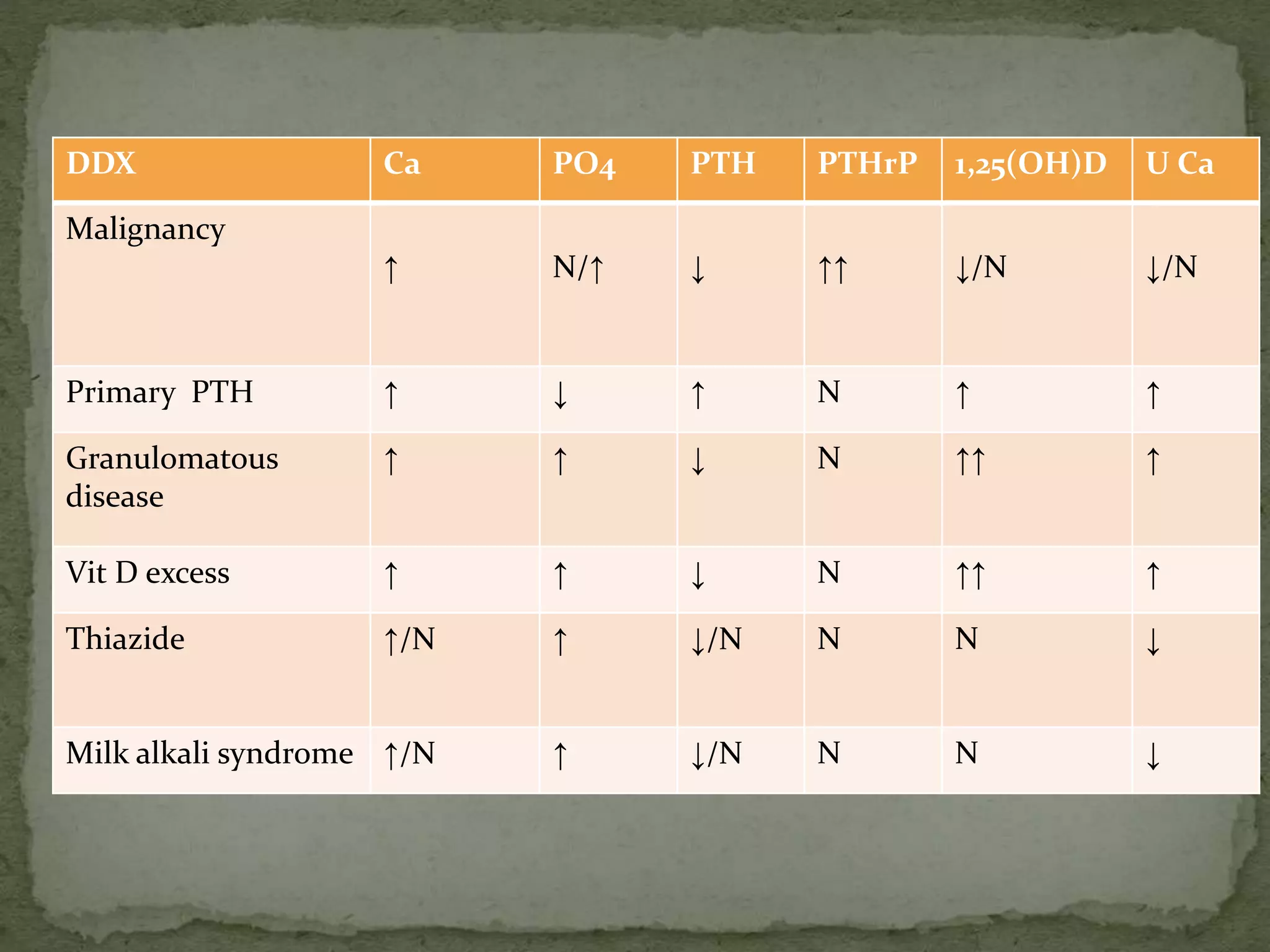
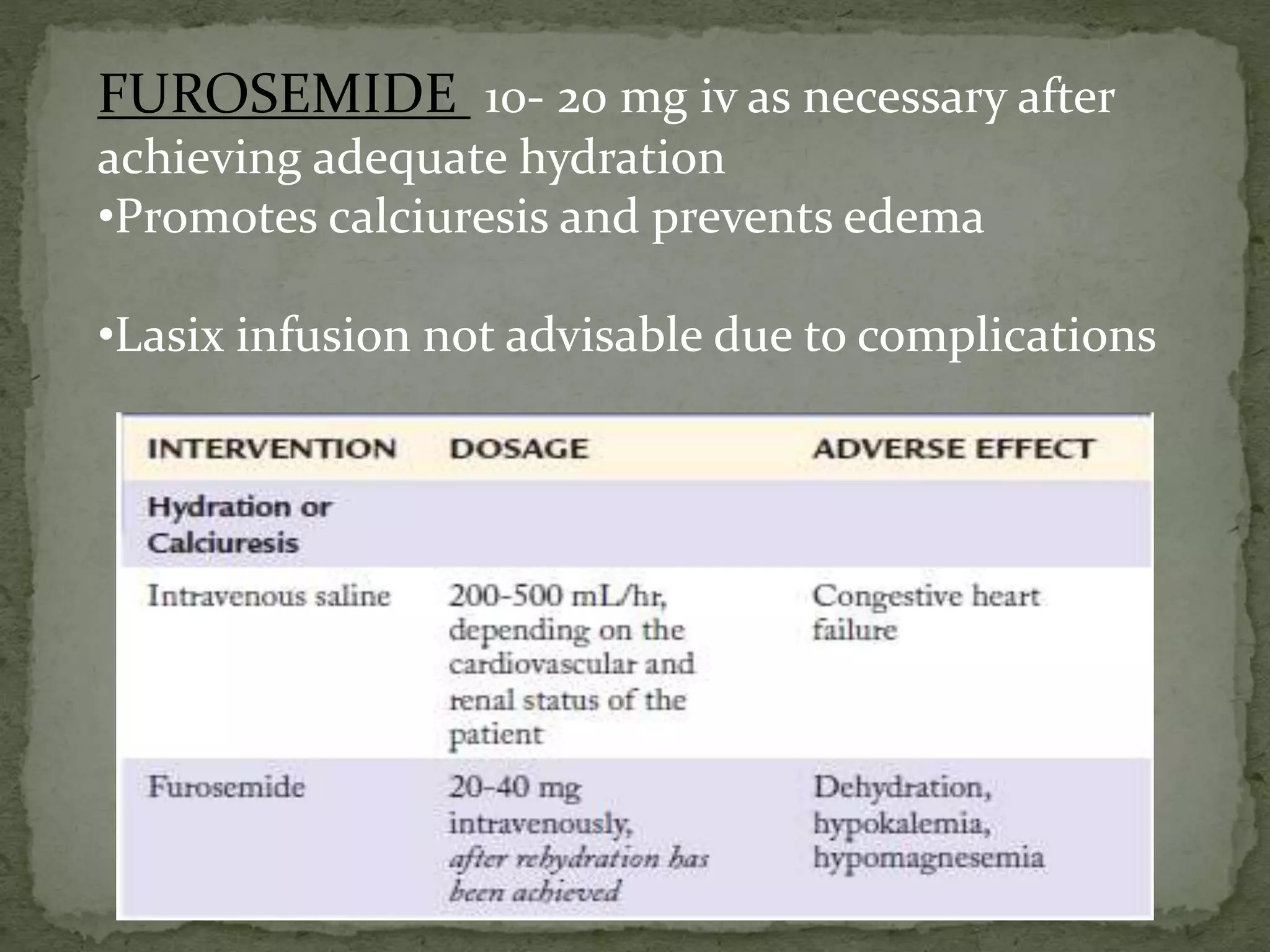
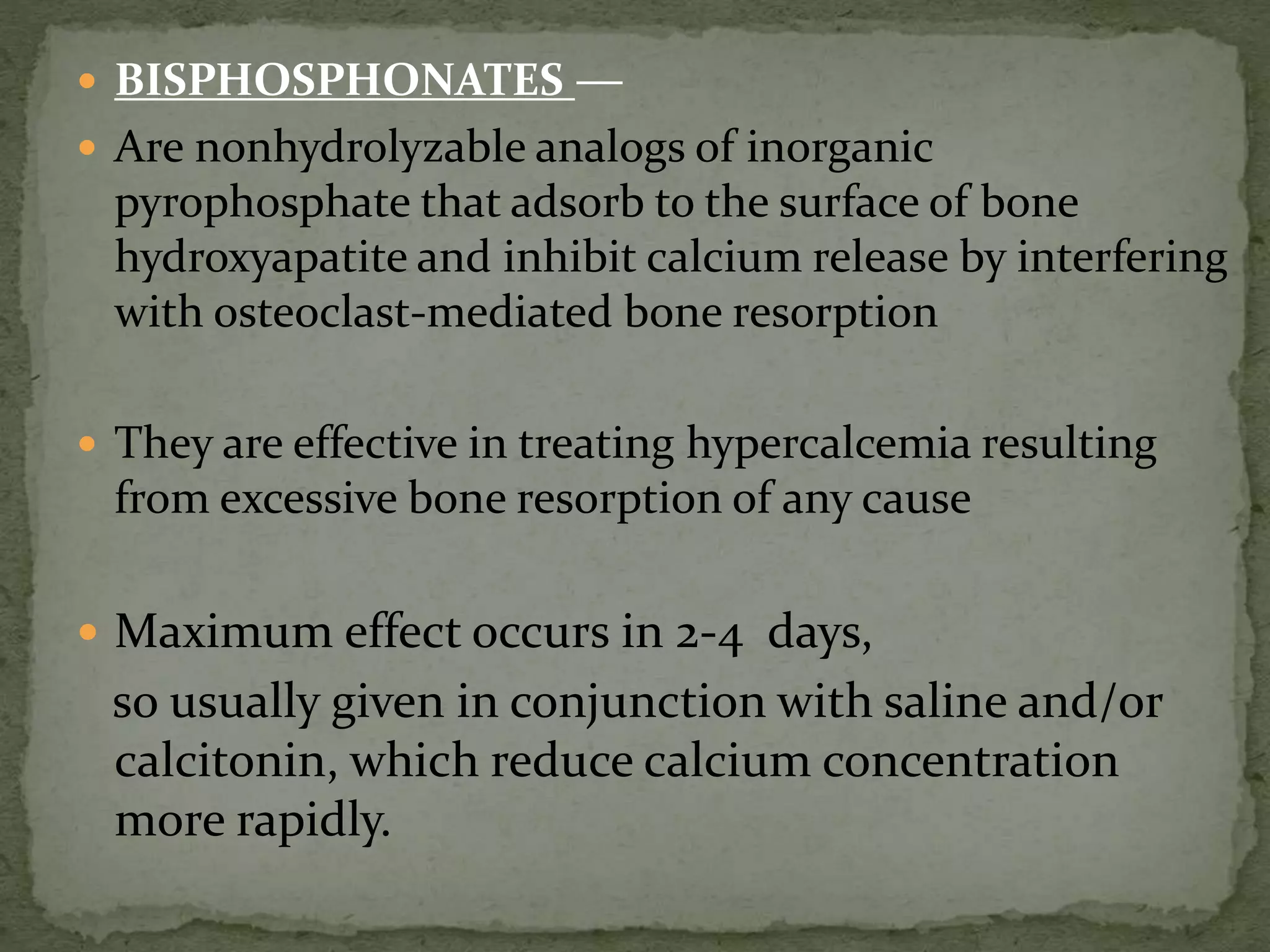
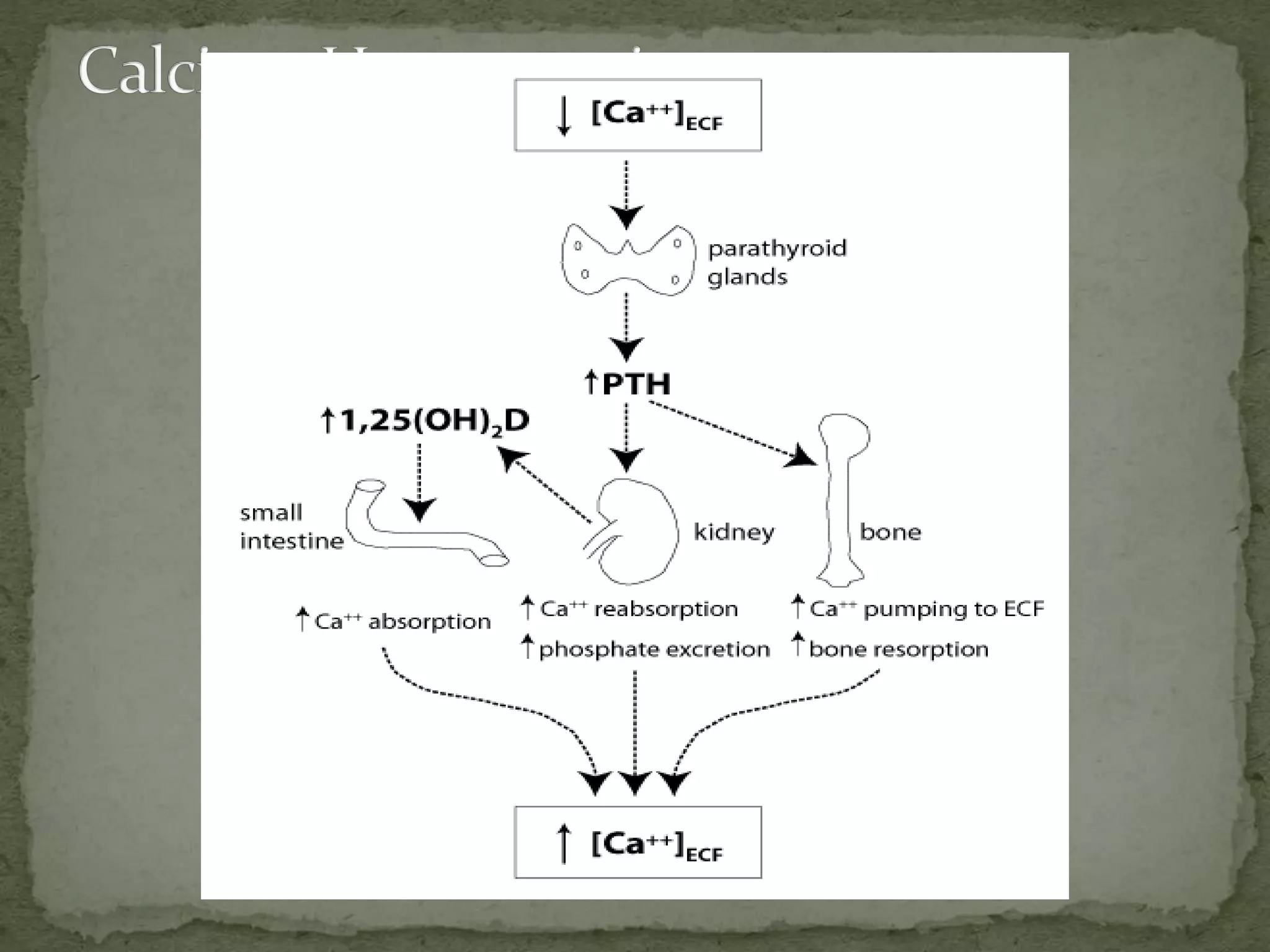
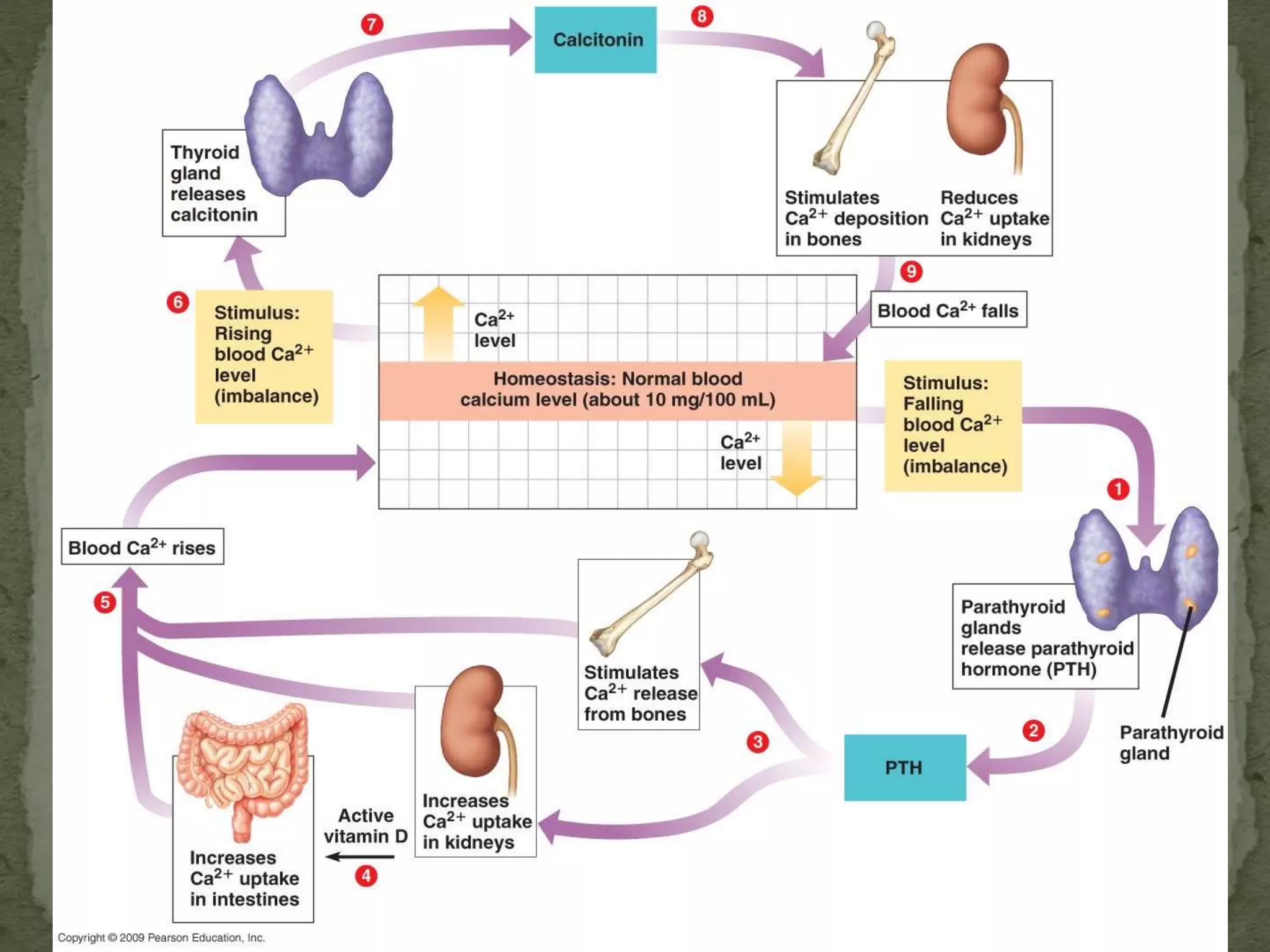
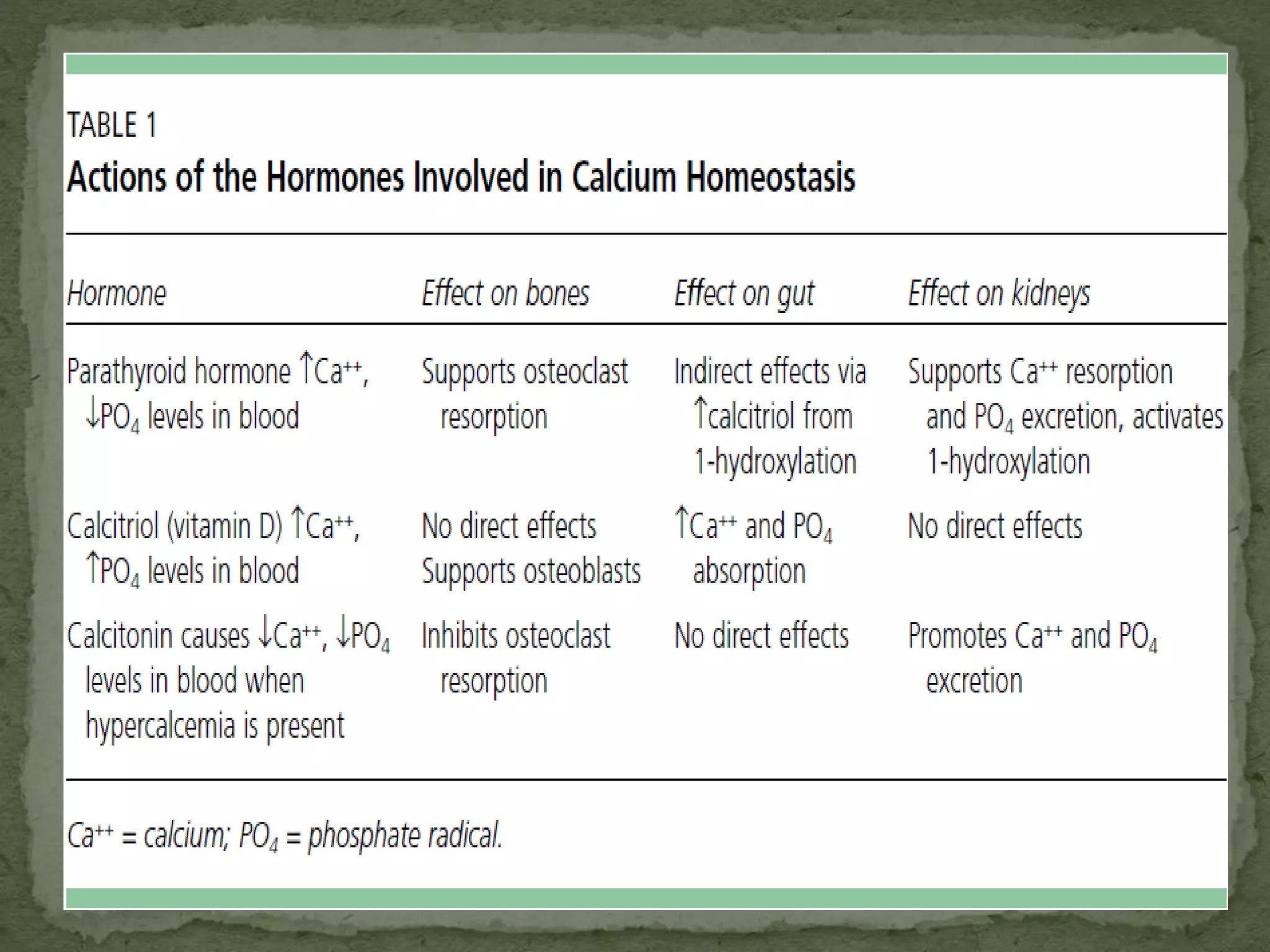
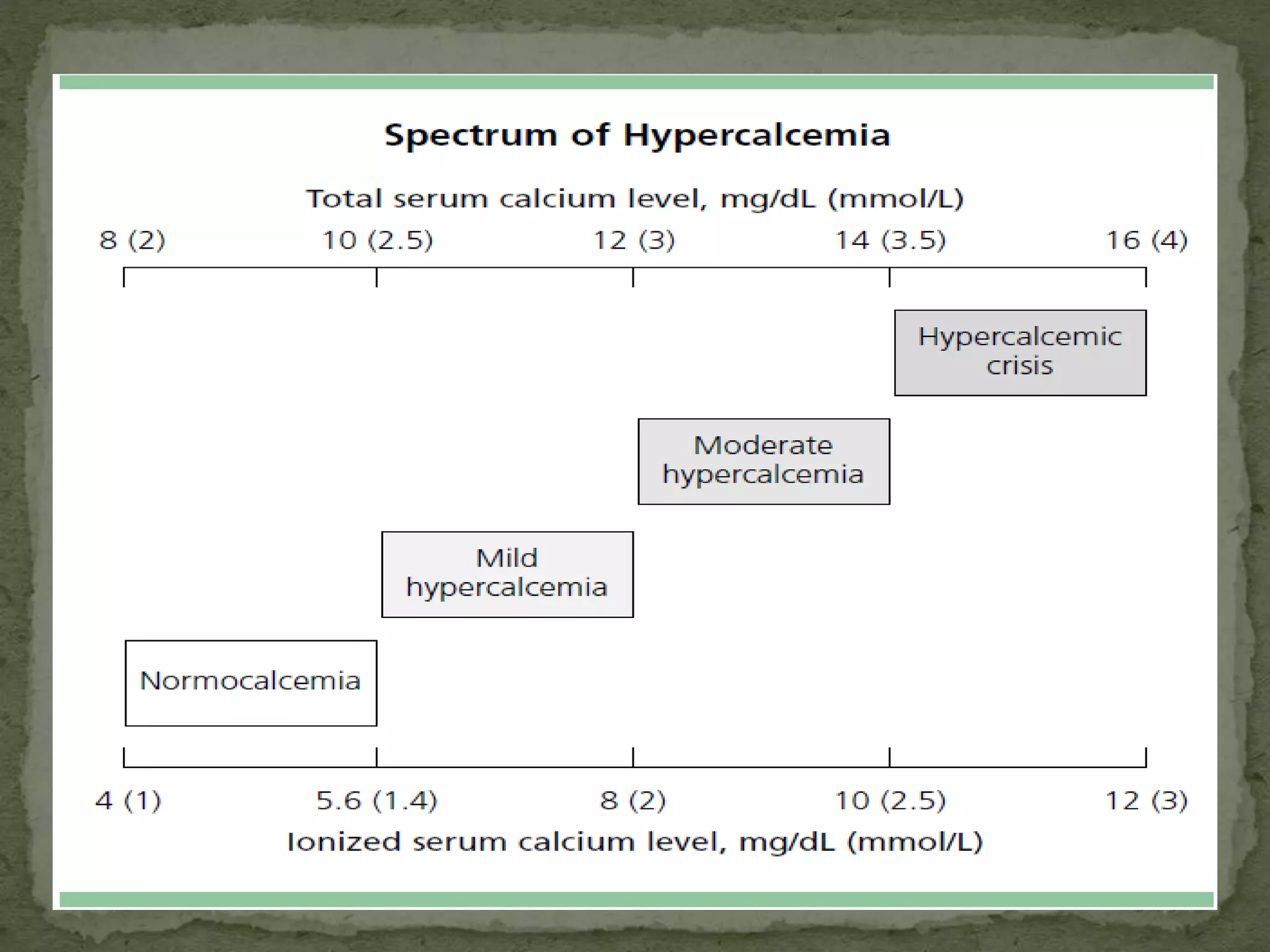
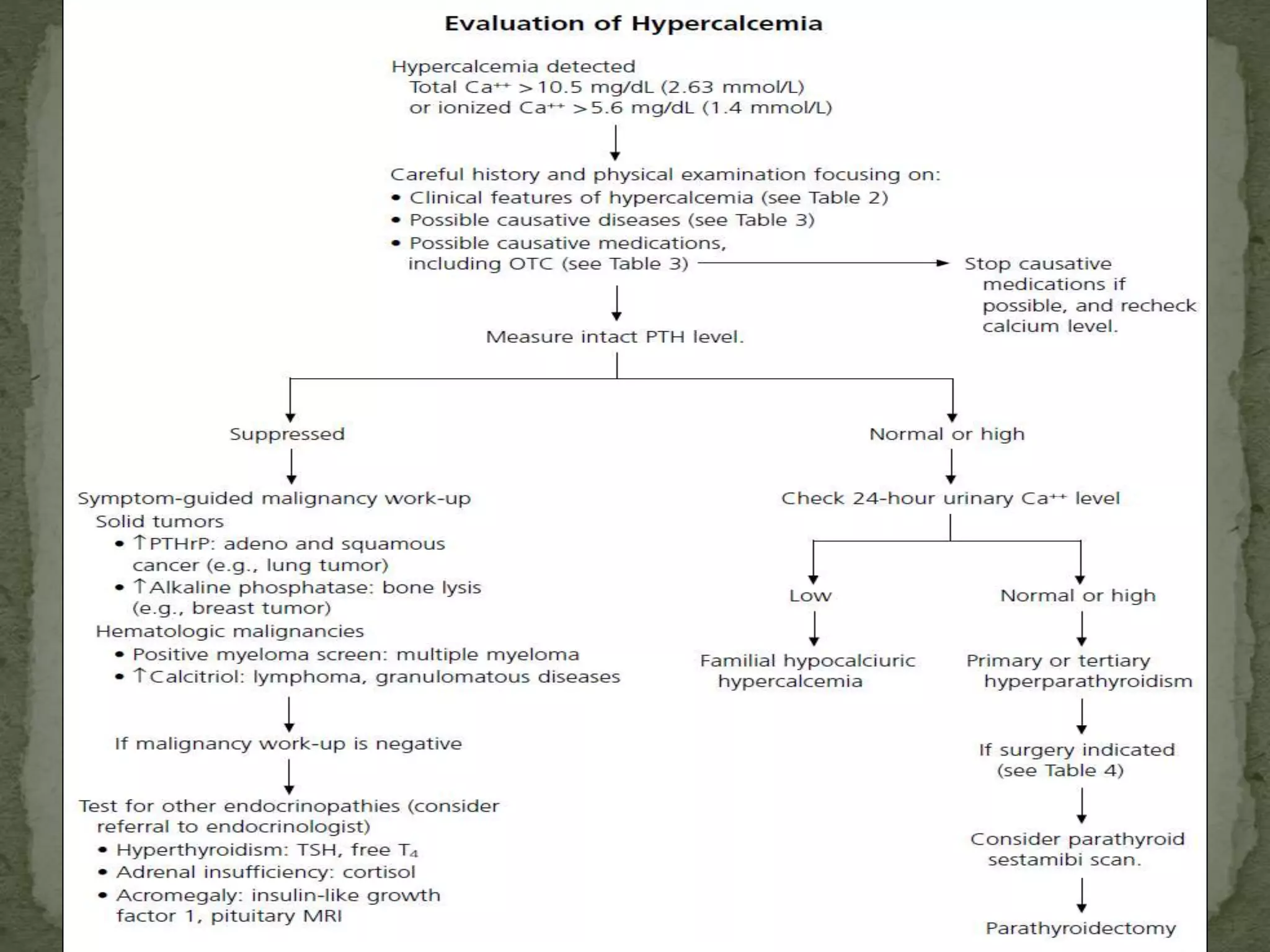
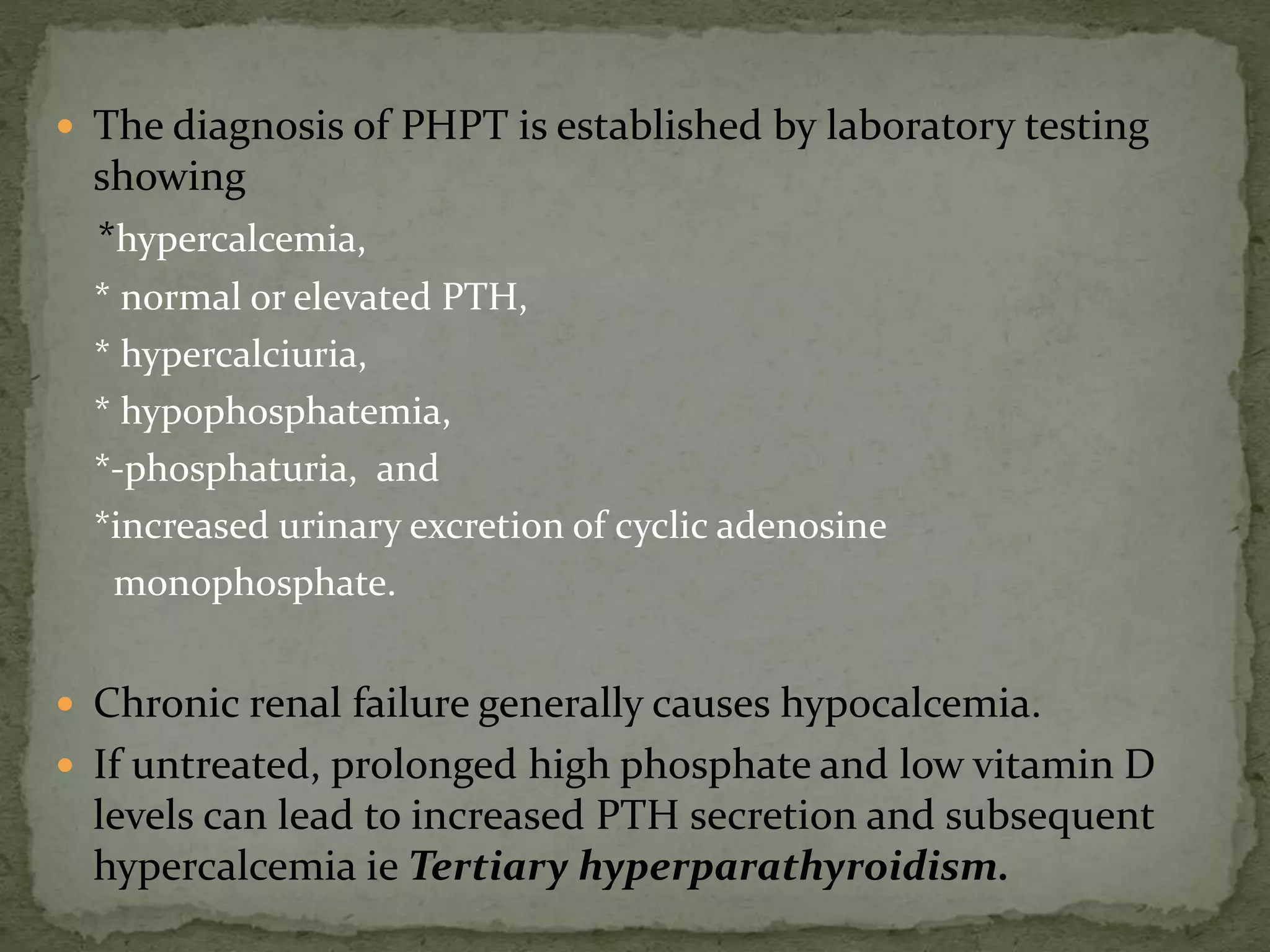
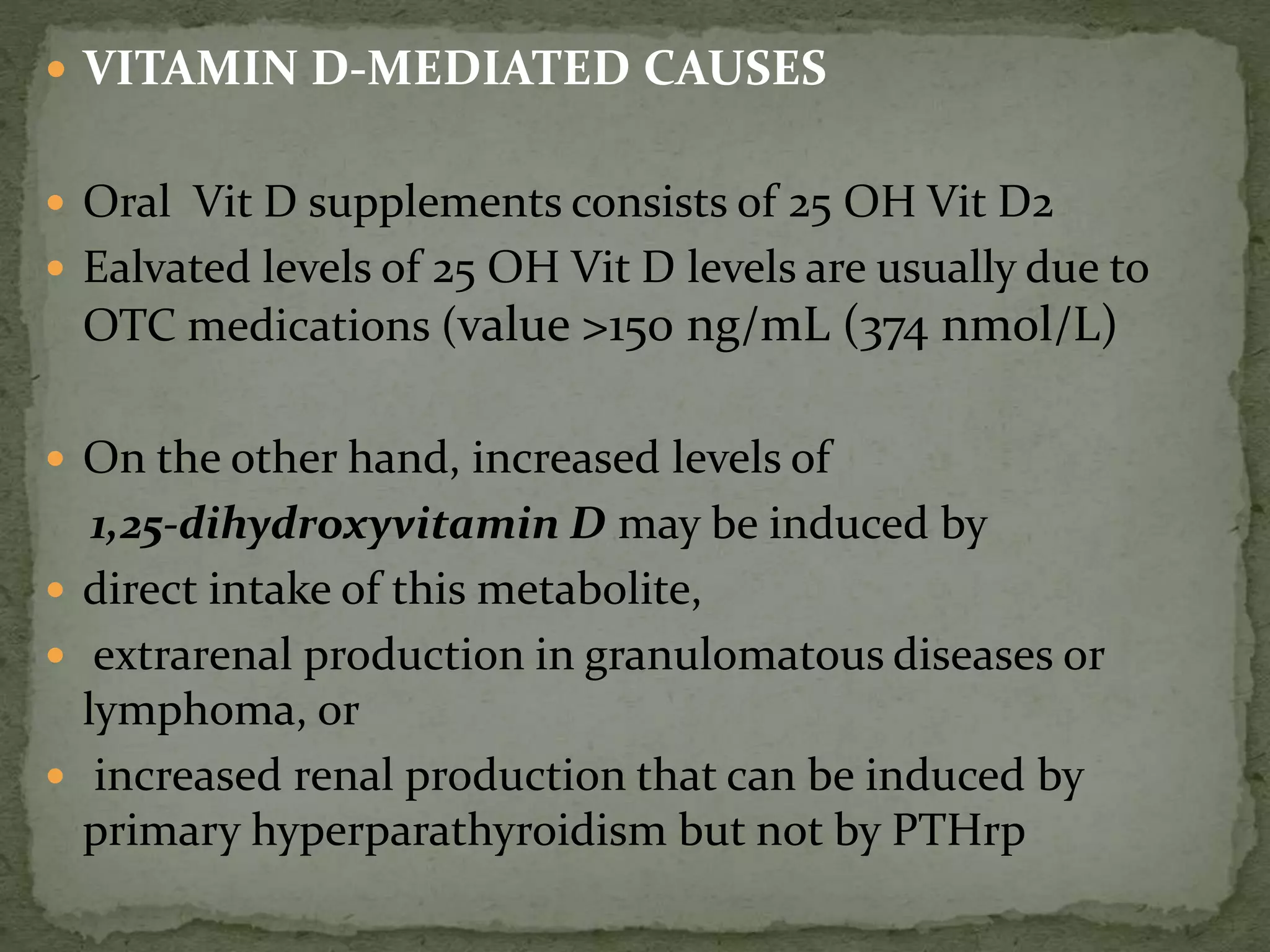
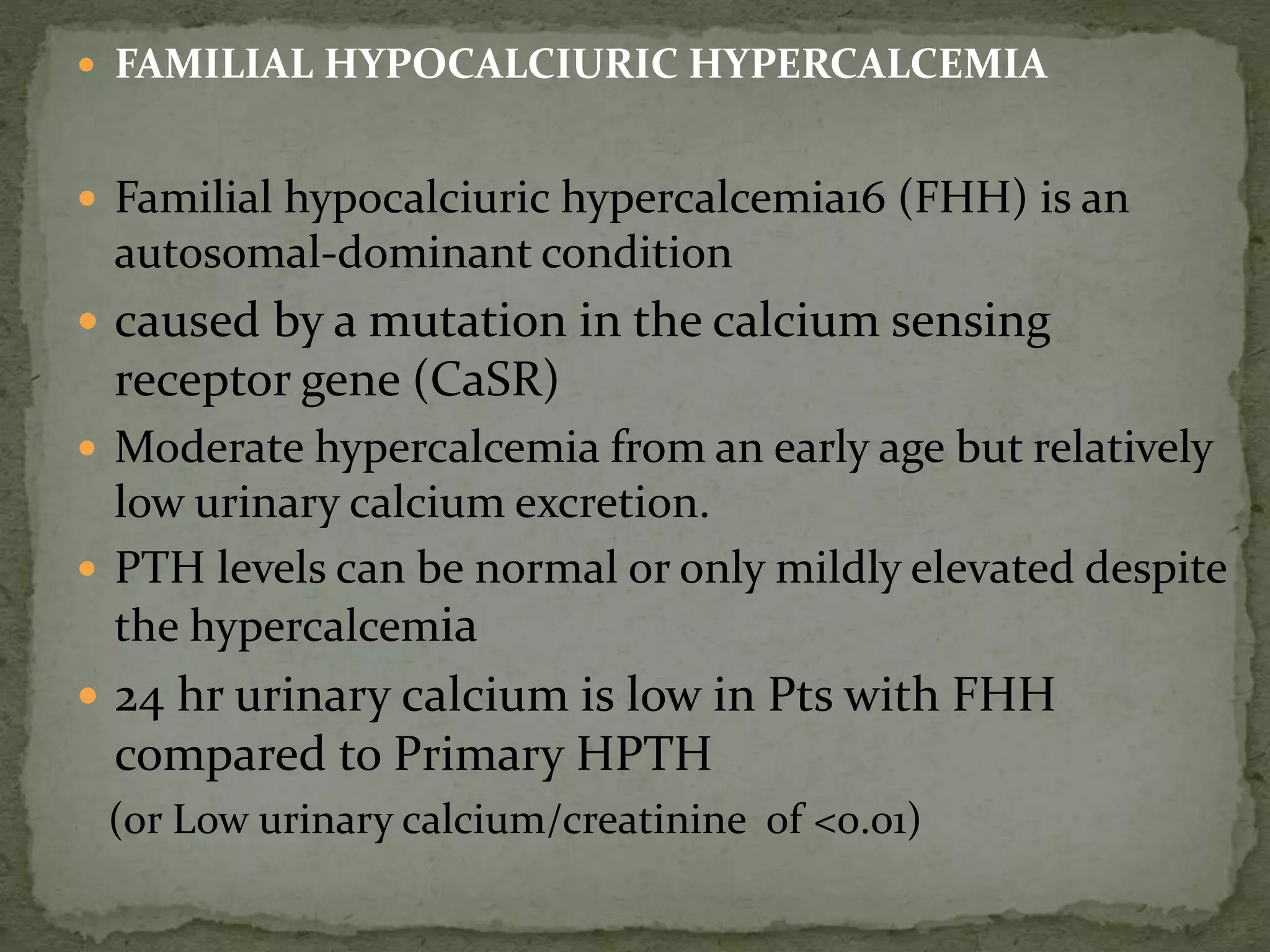
This document discusses calcium homeostasis and hypercalcemia. It notes that calcium is critical for many physiological functions and is mainly stored in bones. Hypercalcemia can be caused by primary hyperparathyroidism, vitamin D excess, certain malignancies, and other conditions. The diagnostic approach involves distinguishing between hyperparathyroidism and hypercalcemia of malignancy based on lab tests. Treatment focuses on rehydration, increasing calciuresis, and decreasing bone resorption or intestinal calcium absorption using medications like calcitonin, bisphosphonates, glucocorticoids, or dialysis depending on the severity of hypercalcemia.




![ As ionized form is the active form of calcium, serum calcium
levels should be adjusted for abnormal serum albumin levels.
Corrected calcium
For every 1-g/dL drop in serum albumin below 4
g/dL, measured serum calcium decreases by 0.8
mg/dL.
Corrected calcium =
Measured Ca + [0.8 x (4 - measured albumin)]
(Calcium in mg/dl; albumin in g/dl)](https://image.slidesharecdn.com/hypercalcemia-140921075939-phpapp02/75/Approach-to-Hypercalcemia-5-2048.jpg)



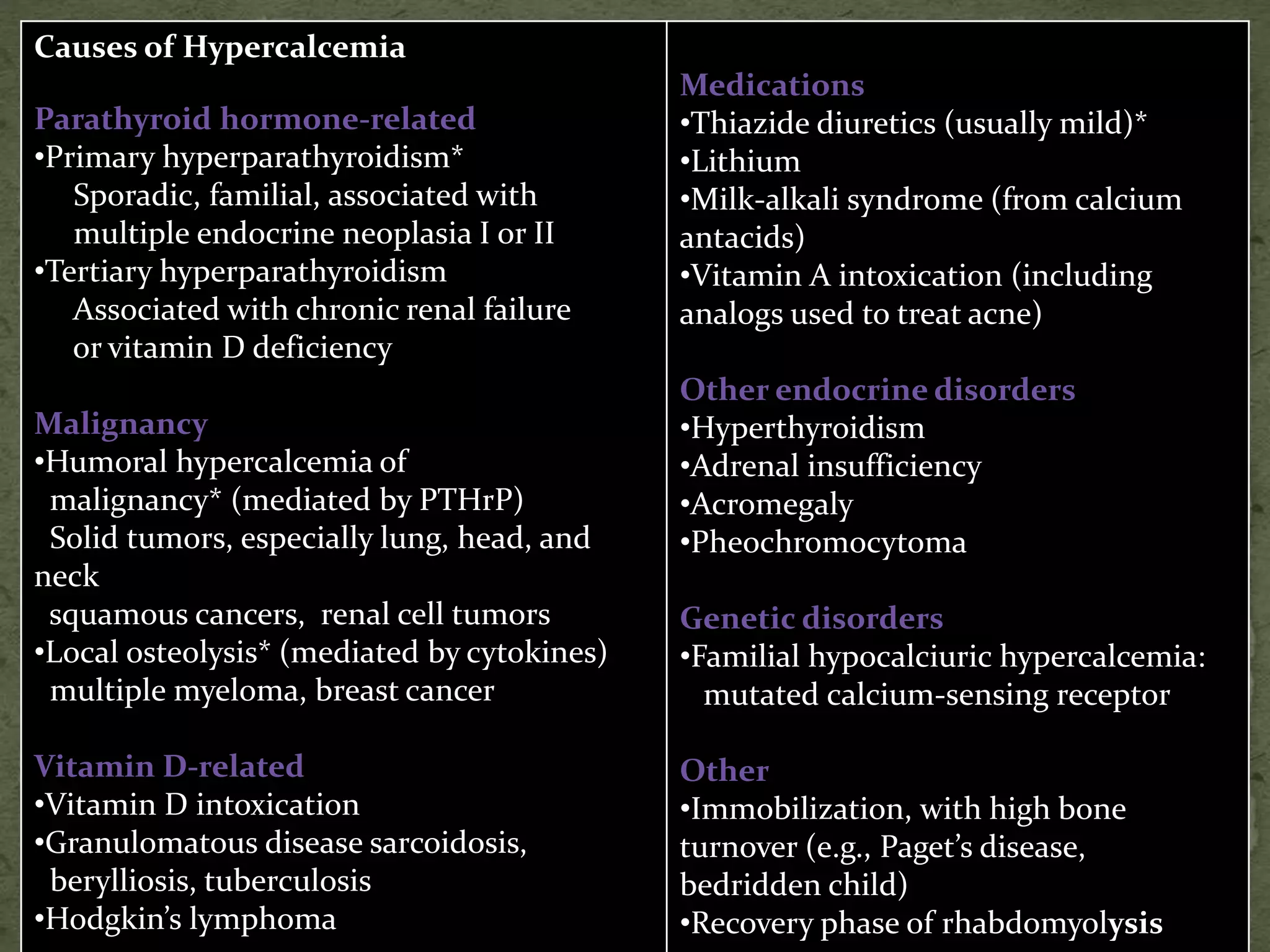






![ Other Causes of Hypercalcemia
Thiazide diuretics:
Enhance ca reabsorption in the distal tubule
↓urinary ca excretion.
Rarely causes Ca in N persons, but lead to Ca in pts
with underlying bone resorption (eg in
hyperparathyroidism)
Mild hypercalcaemia,↓/N PTH
Lithium therapy:
Increased PTH secretion Increasing set point of
PTH, hence higher [Ca] to switch off PTH
Lab inv : high Ca, PTH, low urinary 24(h) calcium](https://image.slidesharecdn.com/hypercalcemia-140921075939-phpapp02/75/Approach-to-Hypercalcemia-25-2048.jpg)