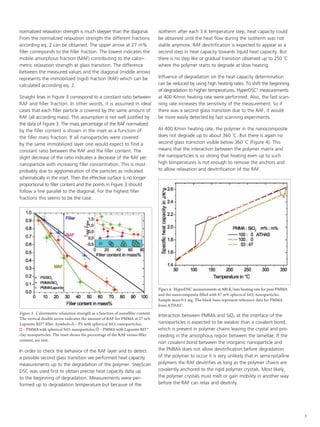
The document discusses the analysis of the rigid amorphous fraction (raf) in polymethyl methacrylate (PMMA) silica nanocomposites using differential scanning calorimetry (DSC) to explore the devitrification behavior. Results indicate that the strong interaction between silica nanoparticles and PMMA prevents raf devitrification prior to polymer degradation, and no evidence of a second glass transition was observed. The findings suggest that in semicrystalline polymers, rigid crystals must melt before raf can relax and devitrify.