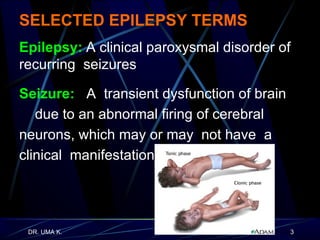
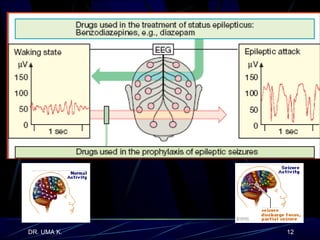
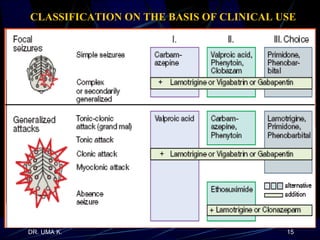
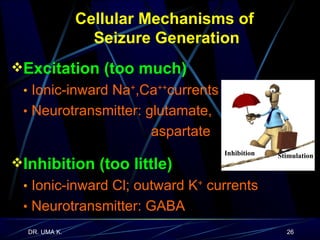
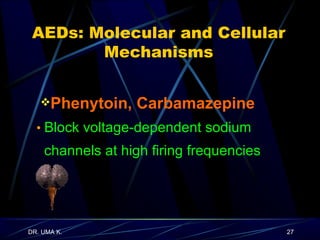
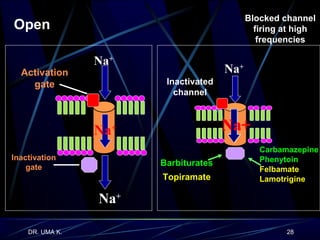
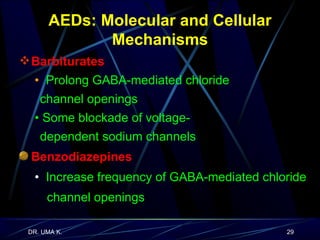
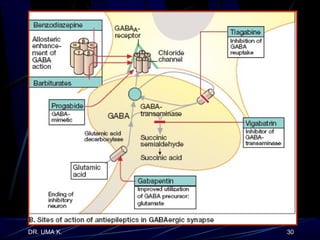
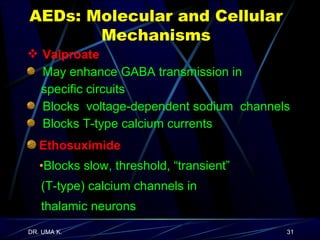
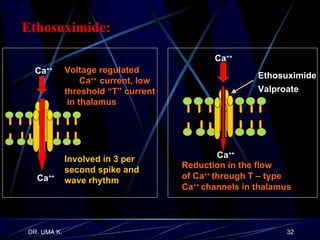
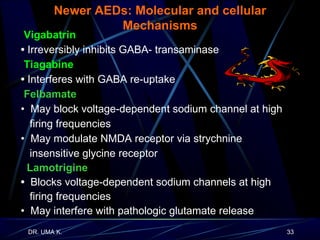
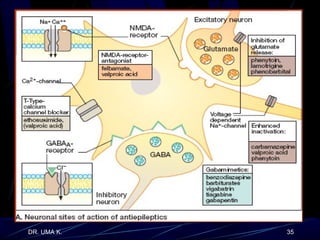
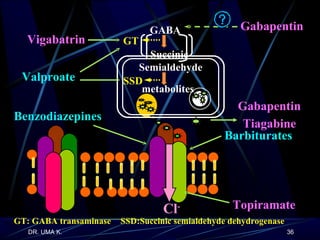
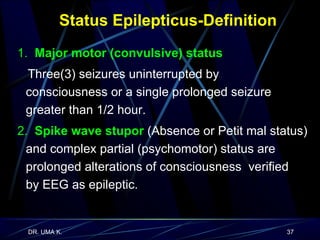
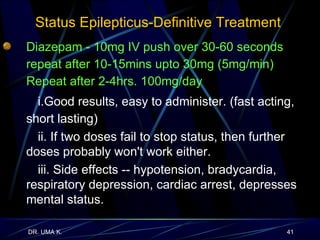
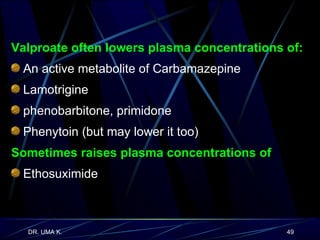
The document defines epilepsy as a group of disorders characterized by chronic recurrent seizures caused by abnormalities in brain electrical activity. It provides definitions for several epilepsy-related terms and seizure types. It also summarizes epidemiological data on incidence and prevalence of epilepsy worldwide. Classification systems for seizures, antiepileptic drugs, and treatment principles and approaches for status epilepticus are outlined.