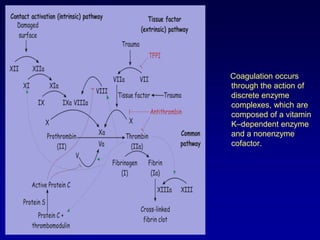
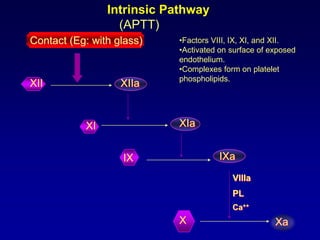
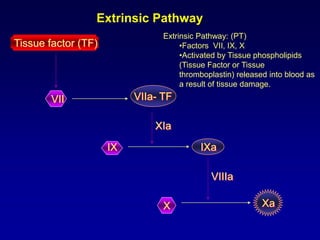
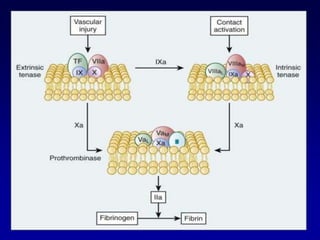
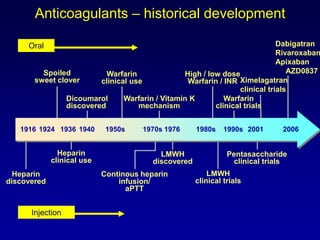
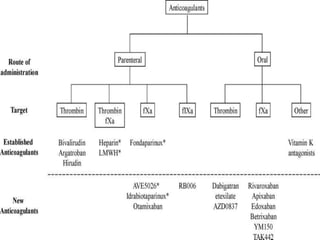
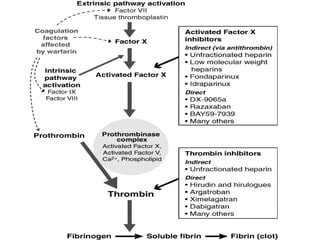
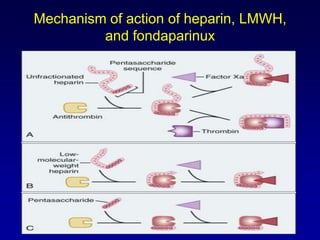
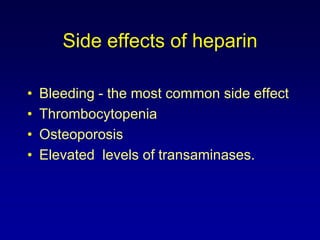
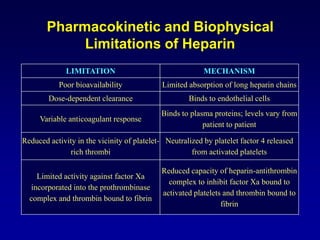
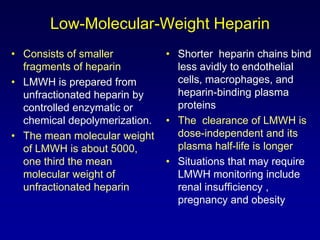
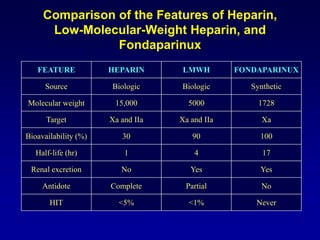
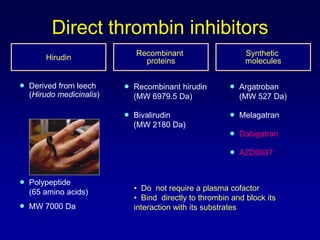
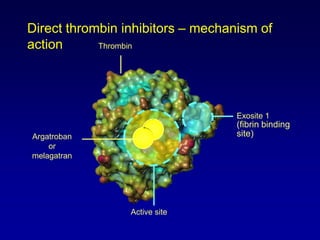
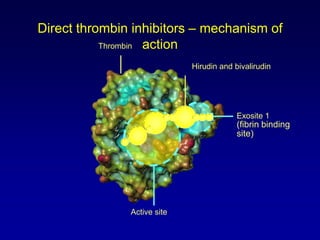
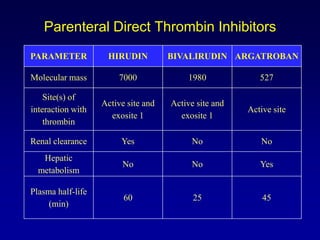
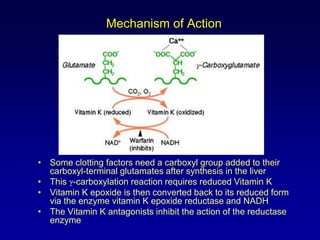
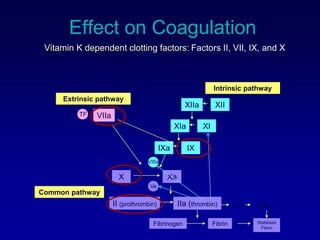
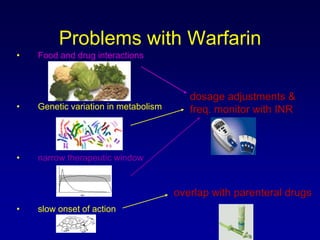
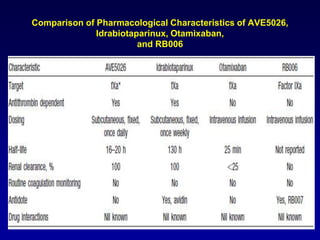
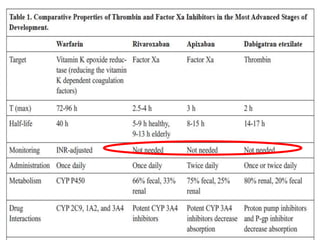
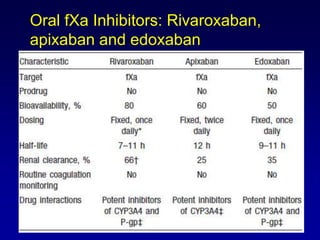
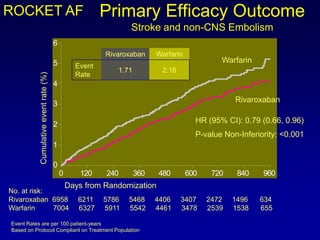
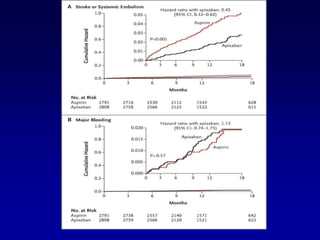
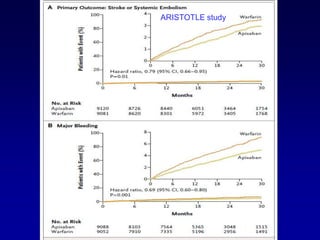
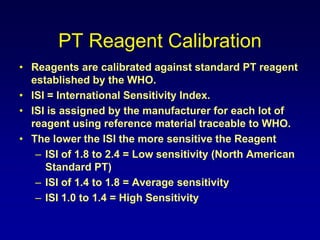
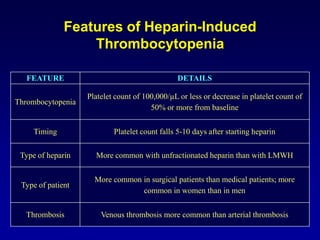
This document summarizes the development of newer anticoagulants, including direct thrombin inhibitors and factor Xa inhibitors. It discusses the limitations of older anticoagulants like heparin, warfarin, and low molecular weight heparins. Newer oral anticoagulants like dabigatran, rivaroxaban, apixaban and edoxaban directly inhibit thrombin or factor Xa and have improved properties over warfarin such as fewer drug and food interactions and more predictable dosing without monitoring. Clinical trials found these newer anticoagulants to be as effective or more effective than warfarin or enoxaparinux for preventing strokes in atrial fibrill