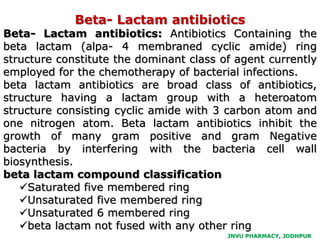
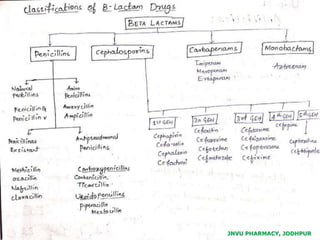
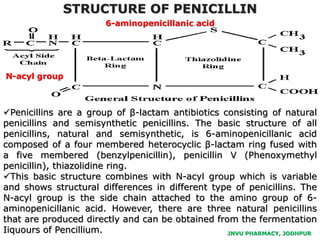
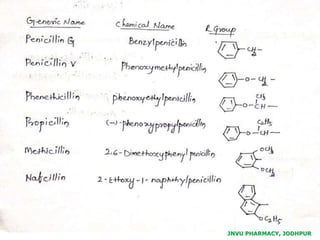
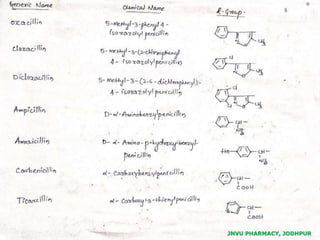
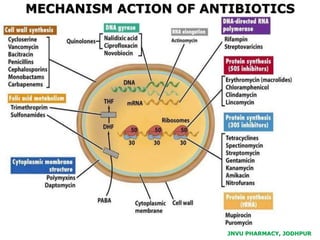
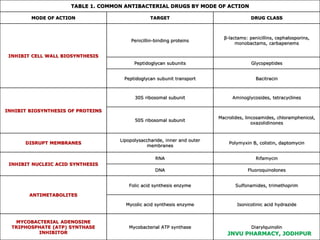
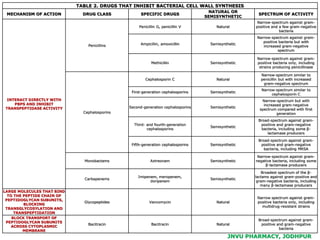
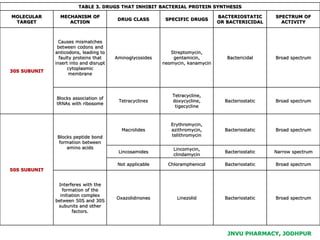
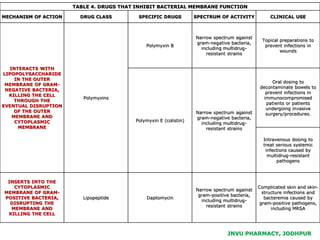
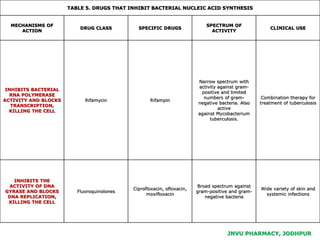
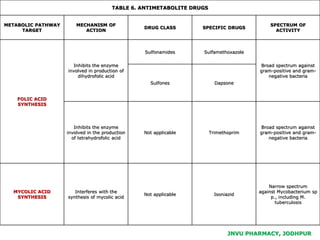
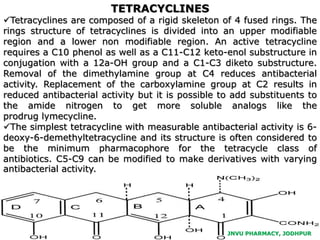
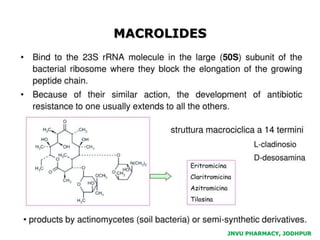
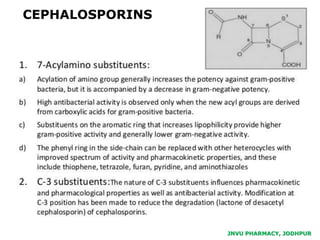
This document discusses antibiotics. It begins by defining antibiotics as chemical compounds that kill or inhibit the growth of bacteria. It then provides a brief history of antibiotics, noting that penicillin was the first antibiotic successfully used to treat bacterial infections. The document goes on to classify antibiotics based on their chemical structure and mechanism of action. It also discusses antibiotic resistance and appropriate antibiotic use.