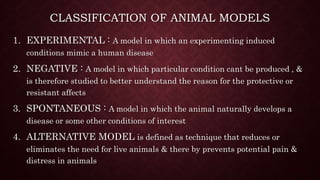
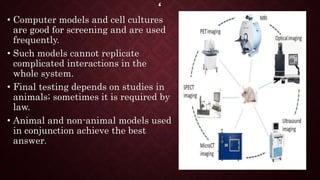
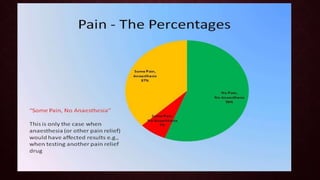
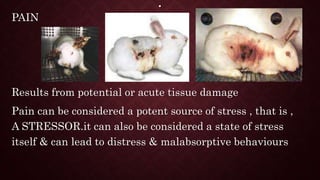
Animal models are important tools in toxicological and biomedical research. Regulations aim to ensure animal welfare while enabling scientific progress. Key points of regulations include:
- Licensing of research facilities and oversight by ethics committees.
- Focus on replacing, reducing and refining animal use (3Rs principle).
- Standards for humane care and treatment of research animals.
- Requirements vary by country but most have adopted versions of the 3Rs and facility licensing with inspections. Self-regulation is common but some places have more direct legal oversight.