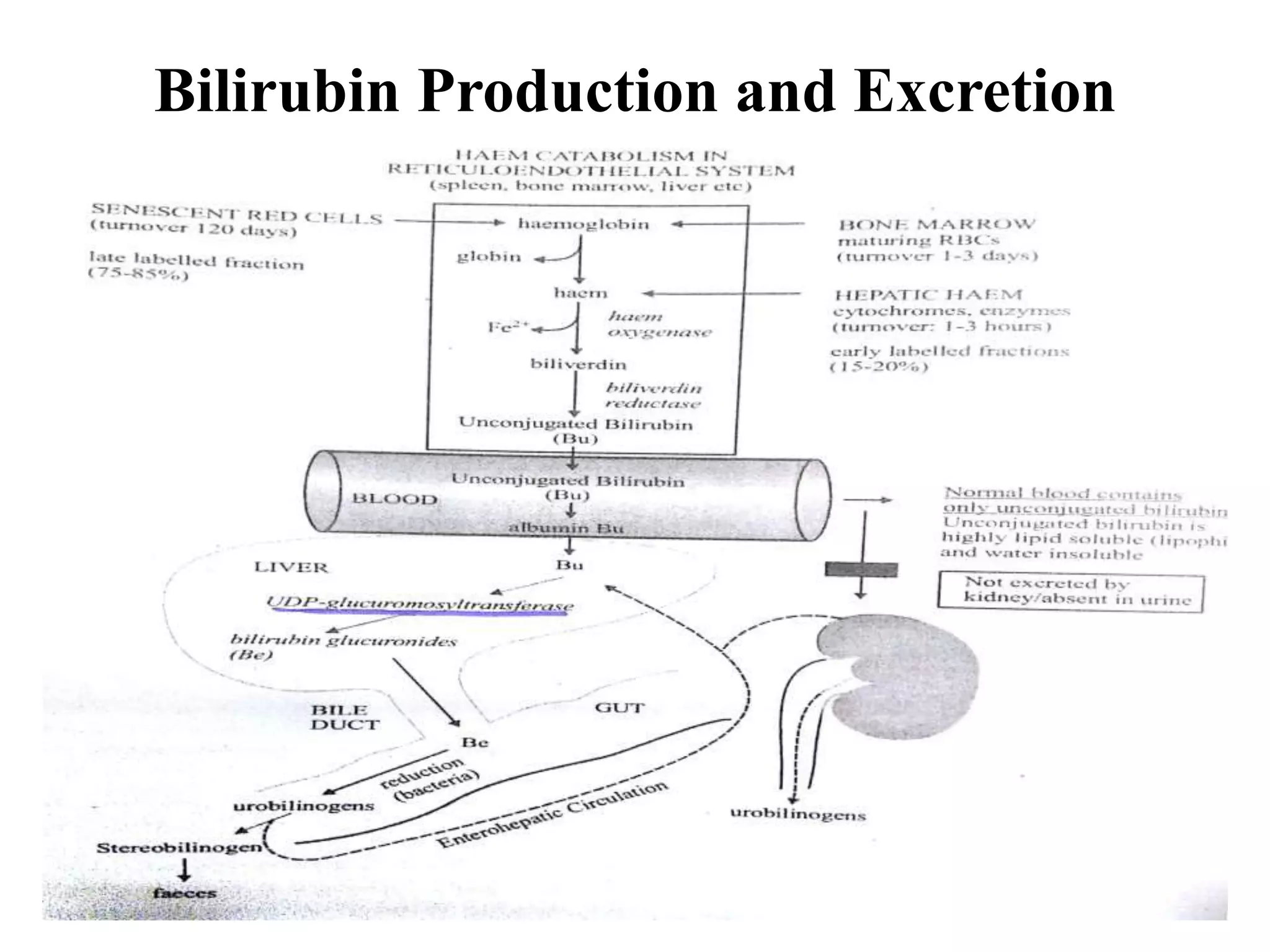
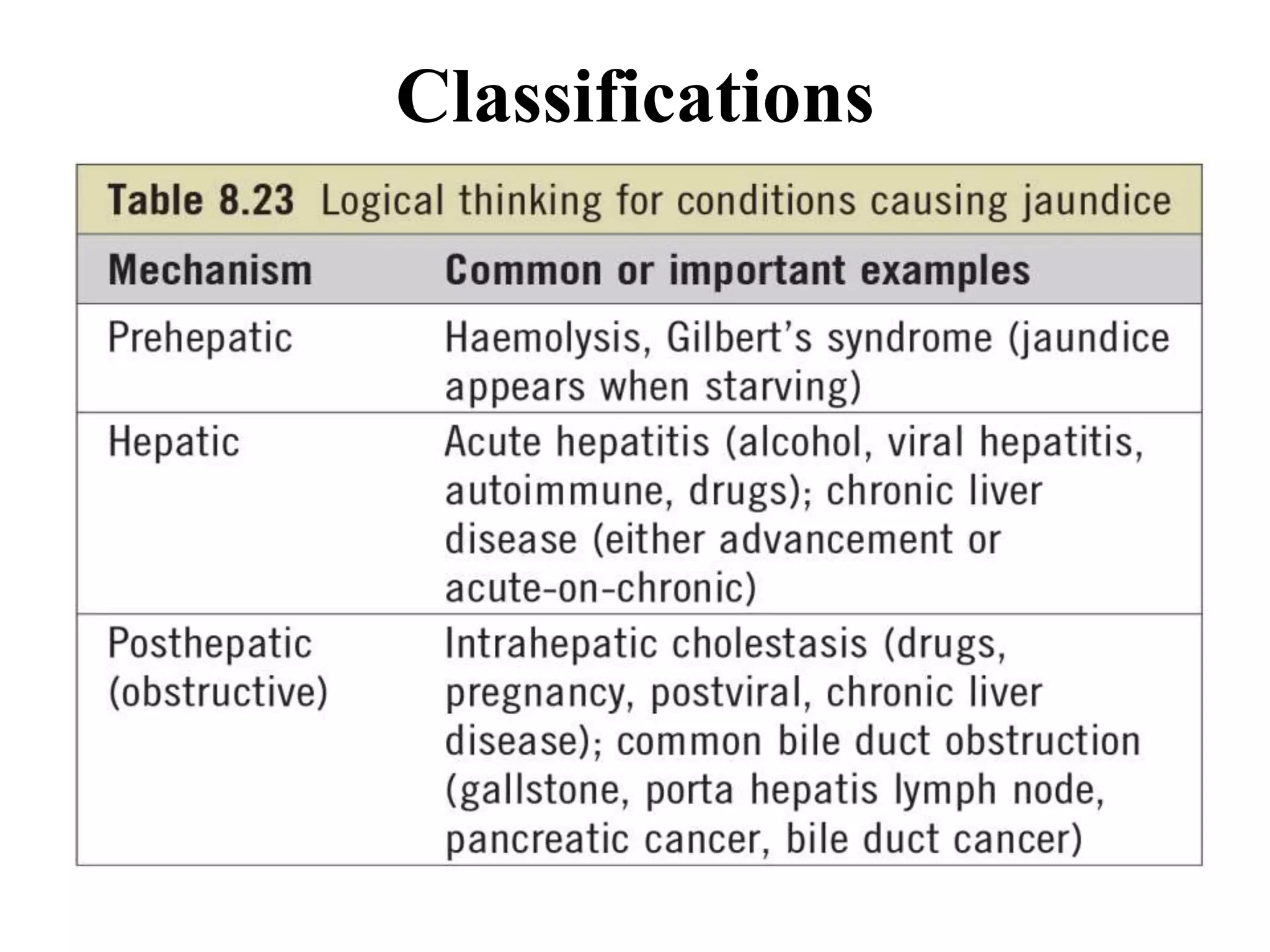
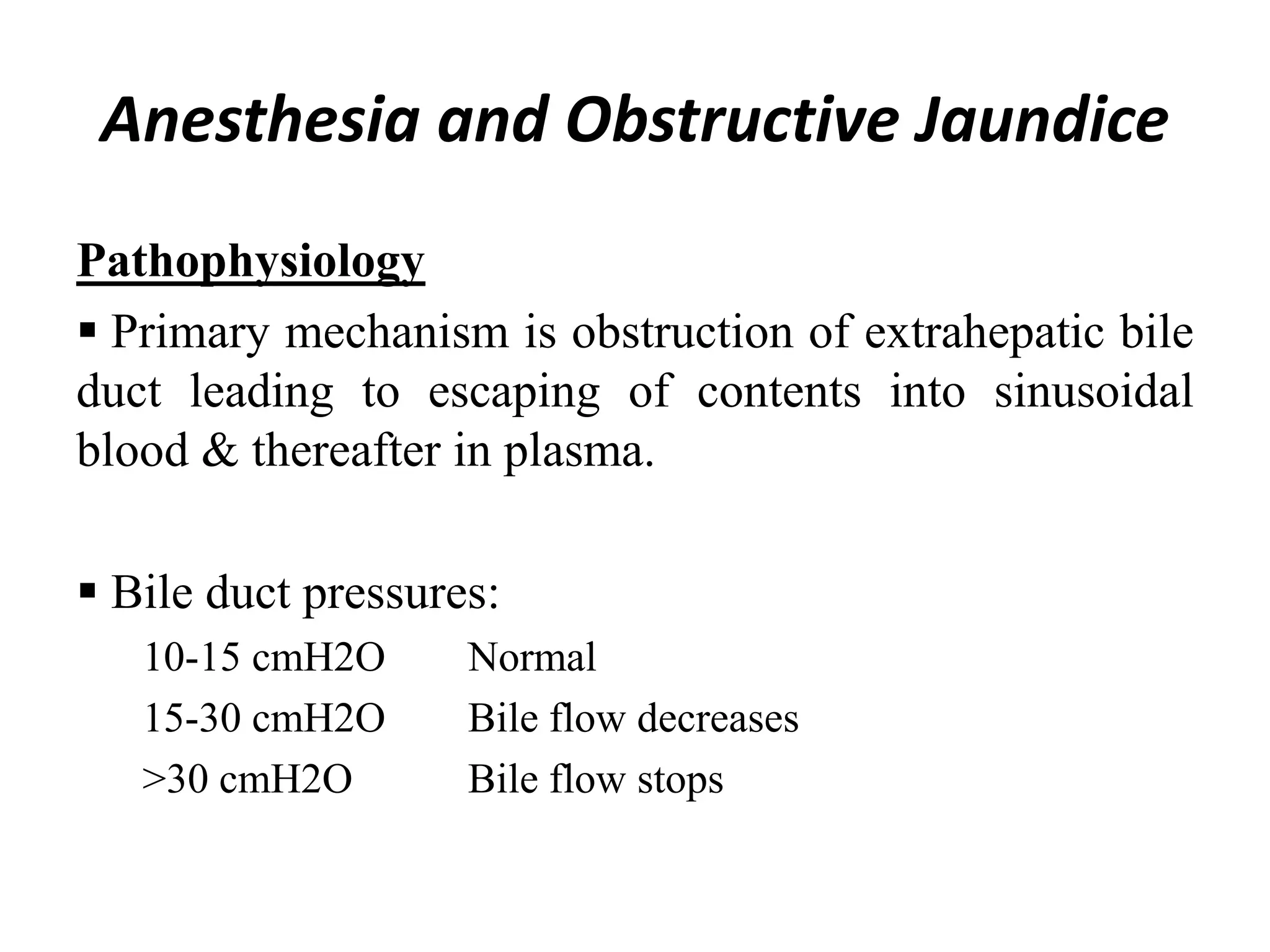
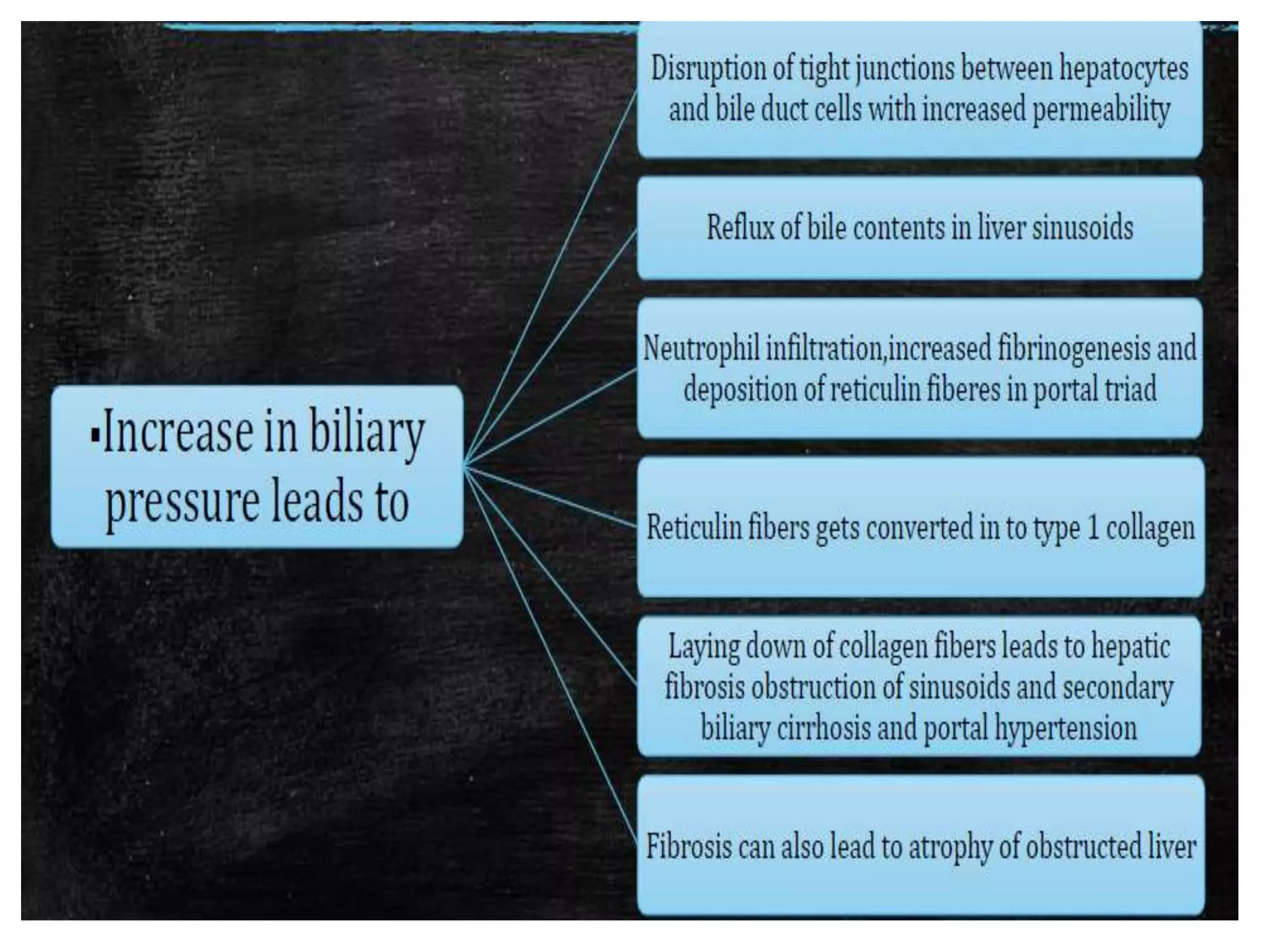
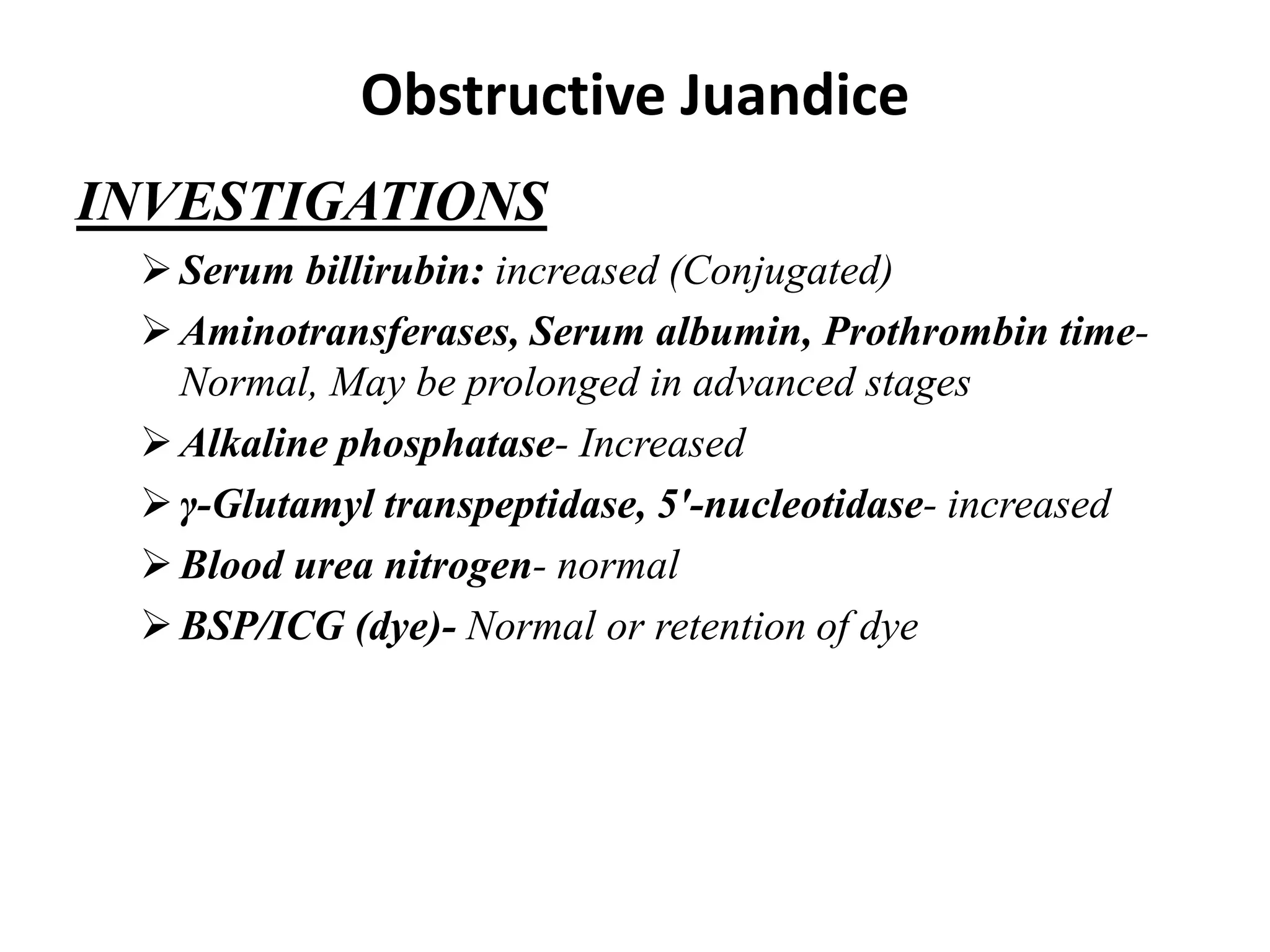
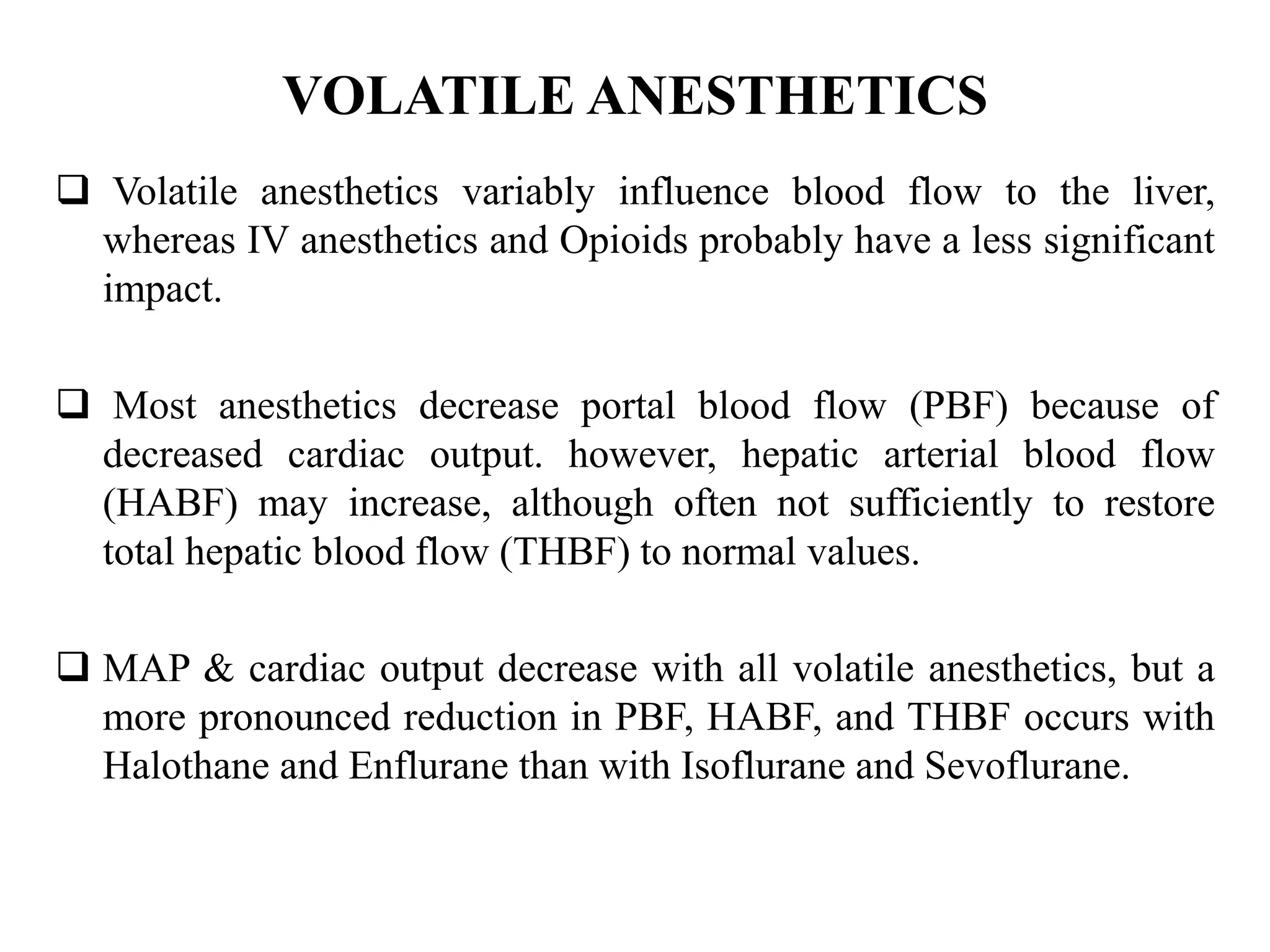
The document provides an overview of obstructive jaundice, detailing its causes, symptoms, and physiological implications. It discusses the liver's crucial functions, the composition and role of bile, and the effects of various anesthetics on hepatic function. The text emphasizes the need for preoperative optimization and careful management in patients with liver dysfunction to minimize complications during anesthesia.