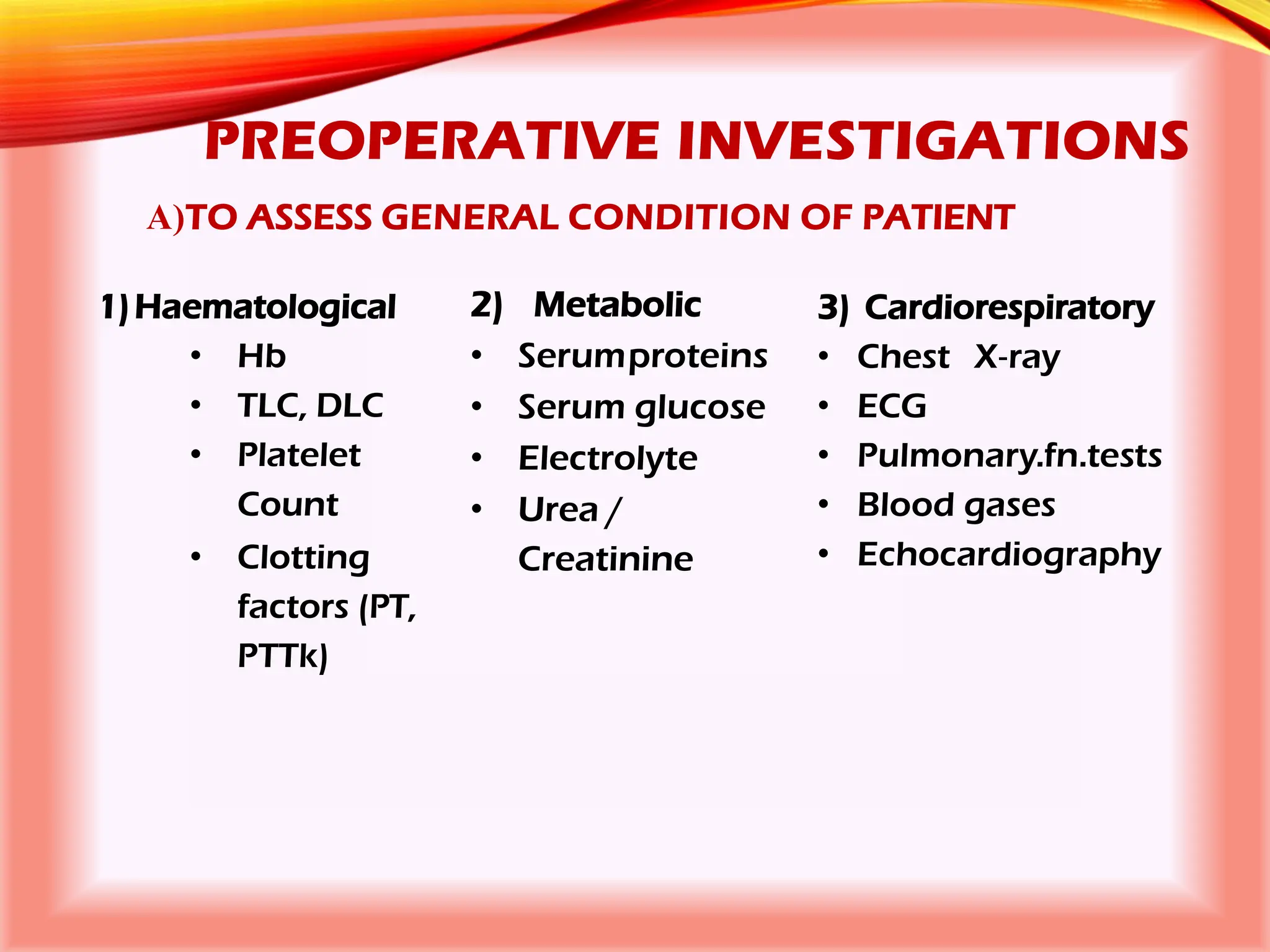
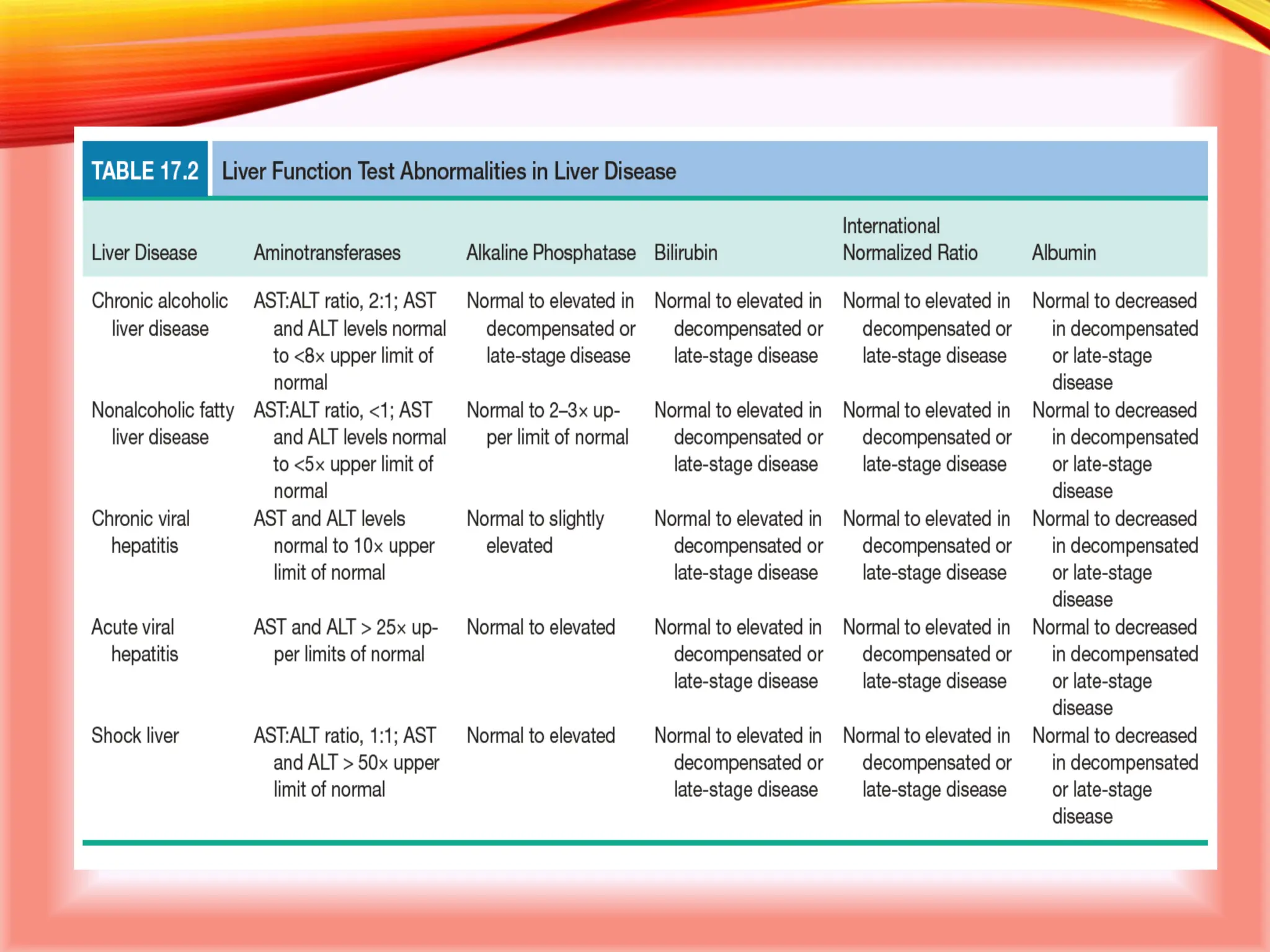
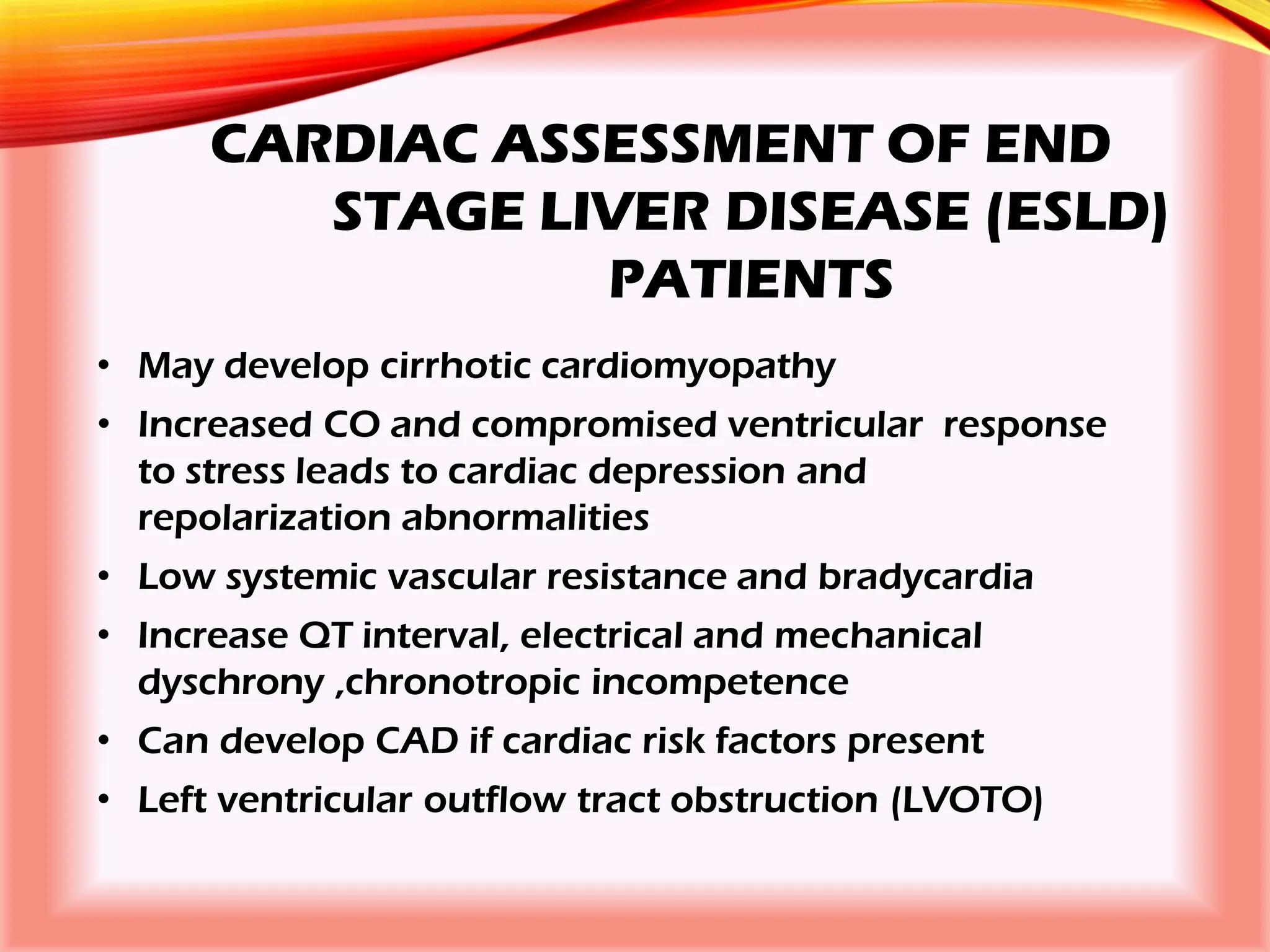
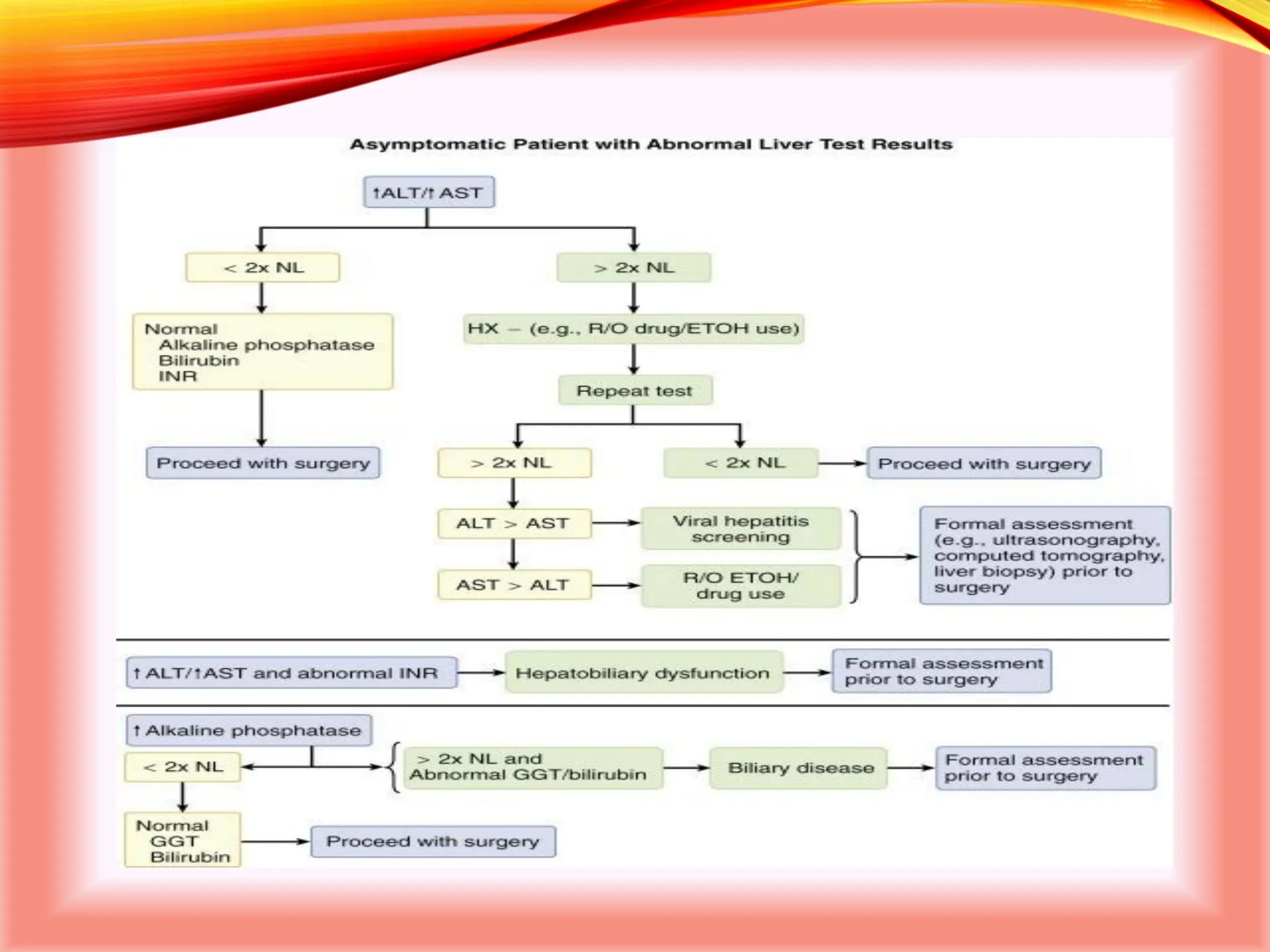
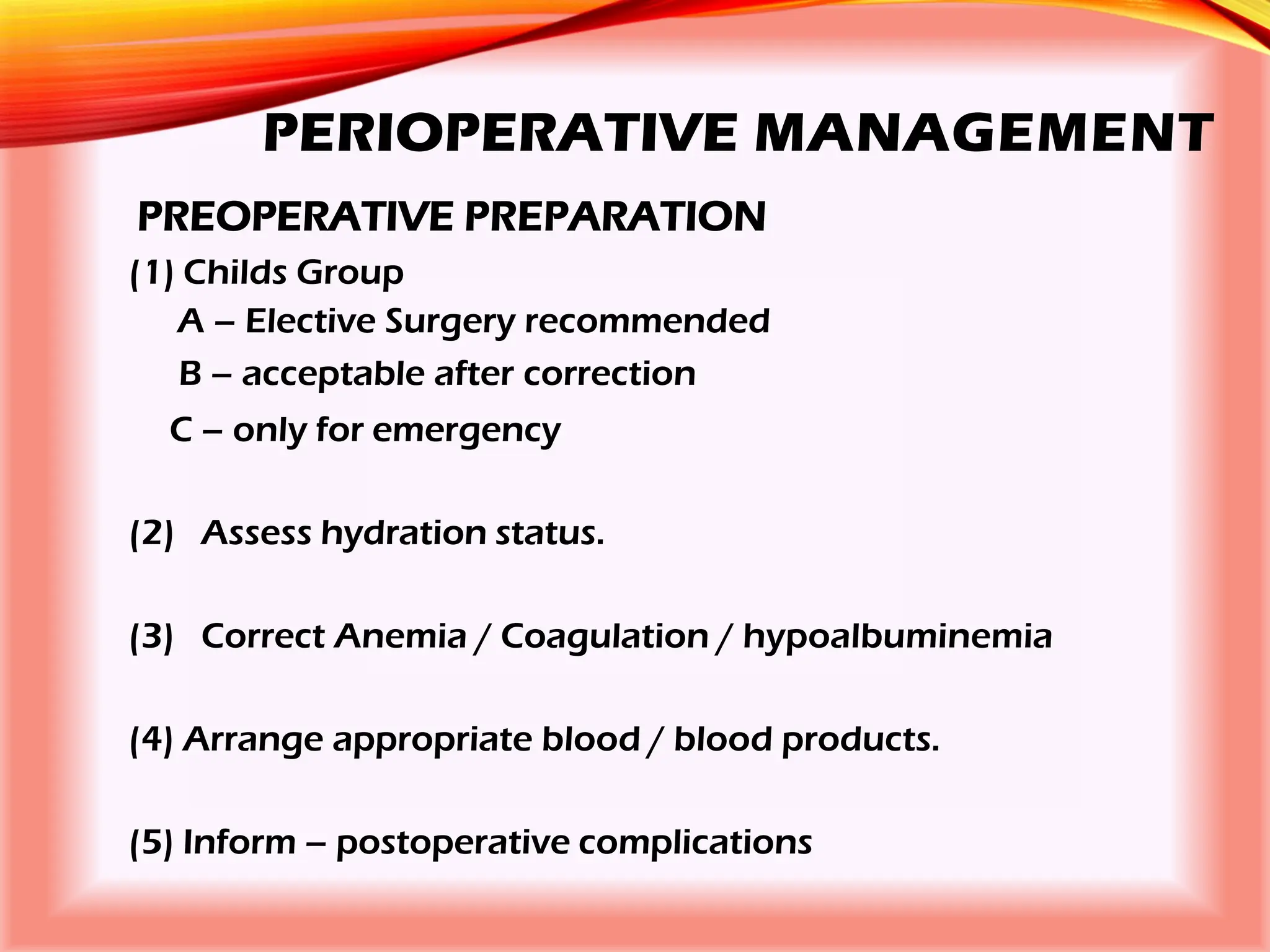
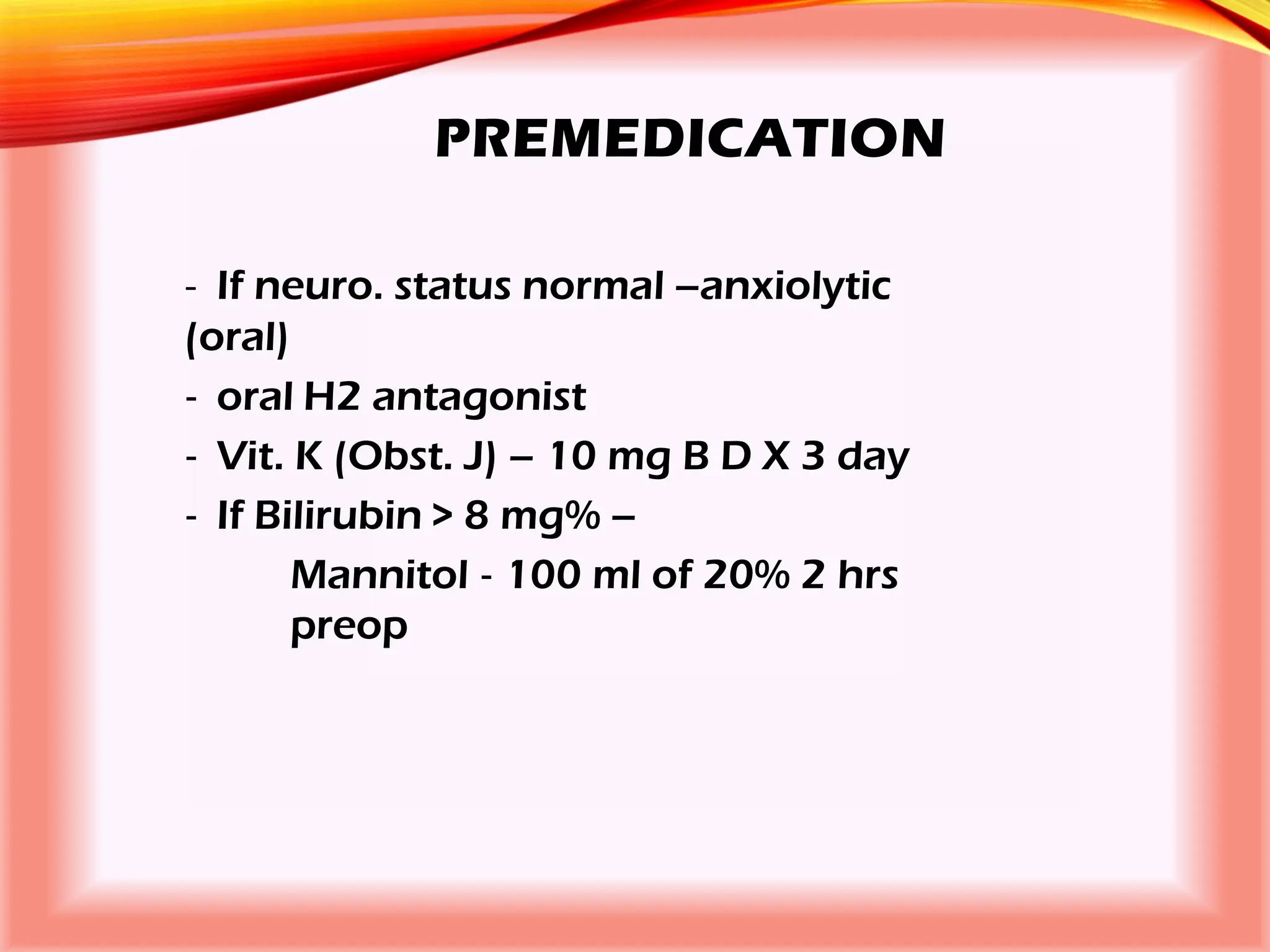
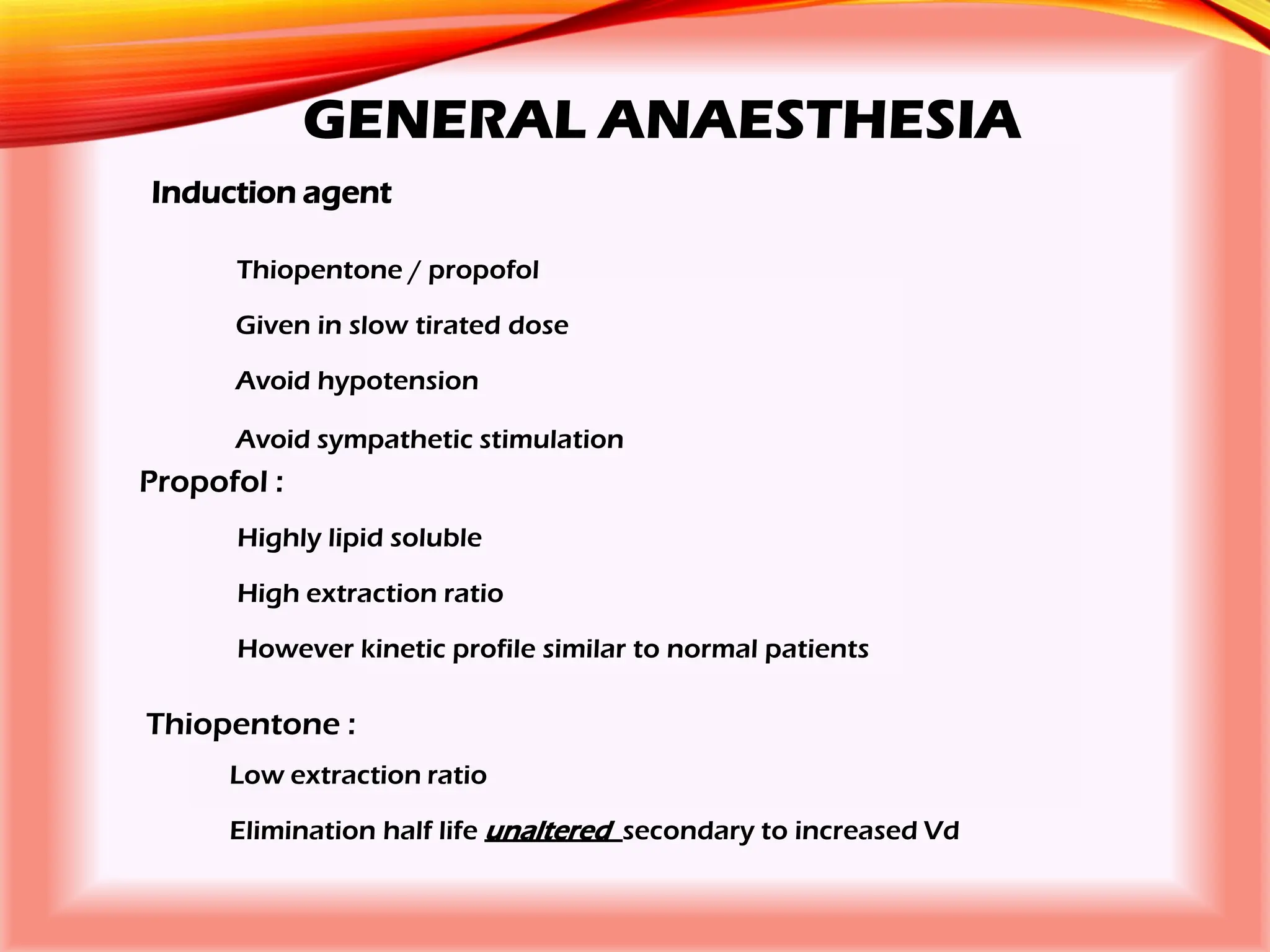
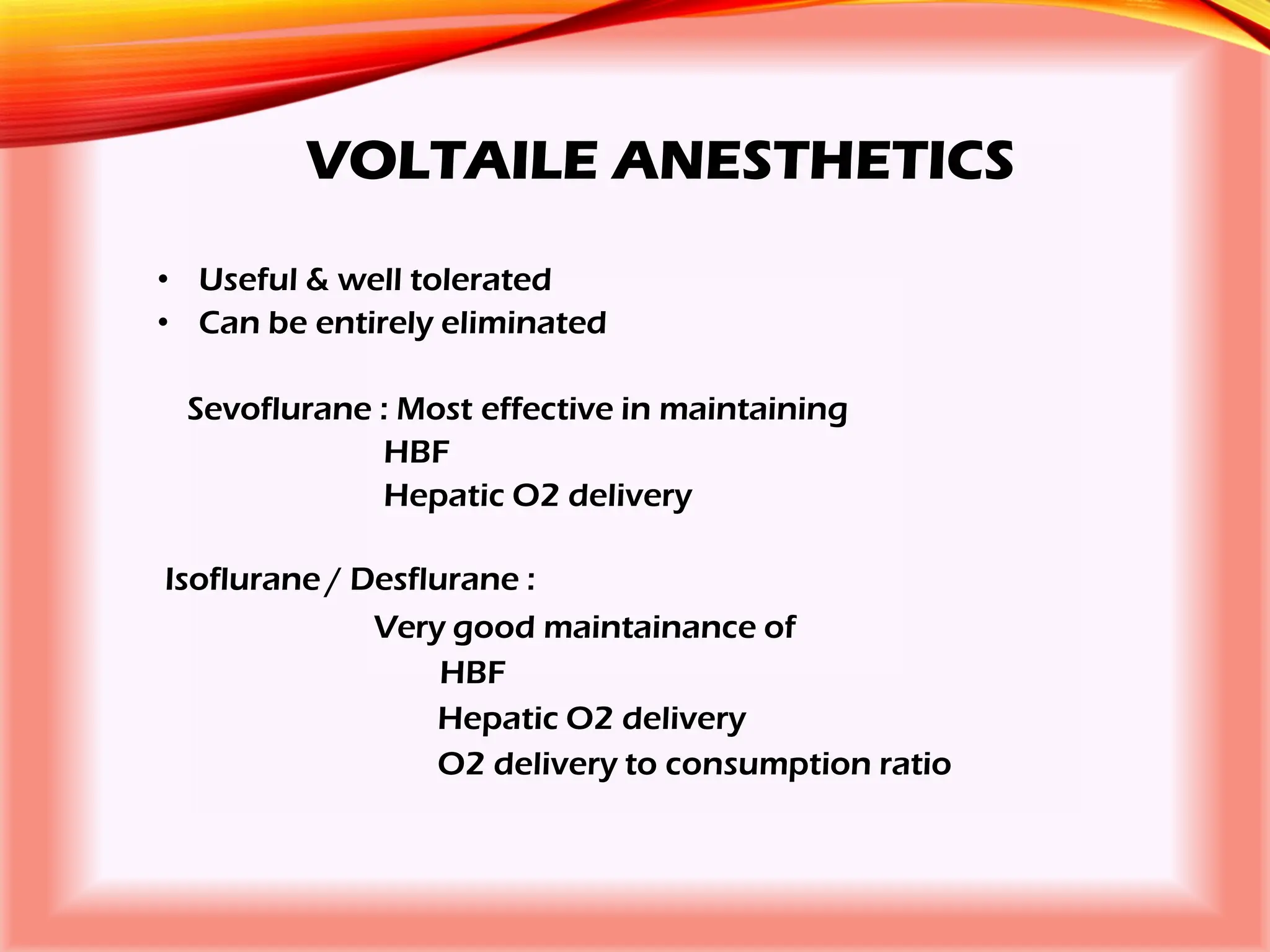
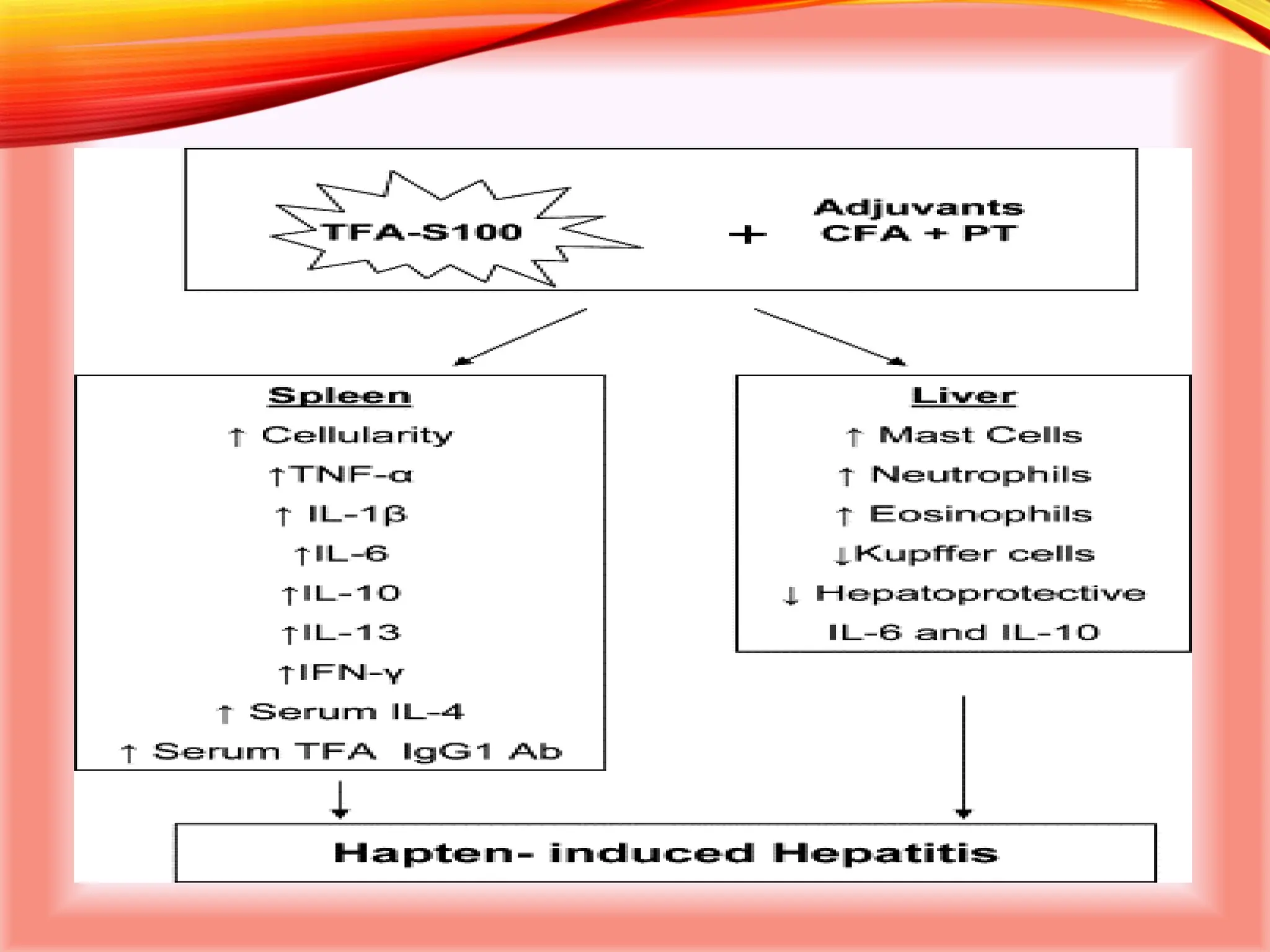
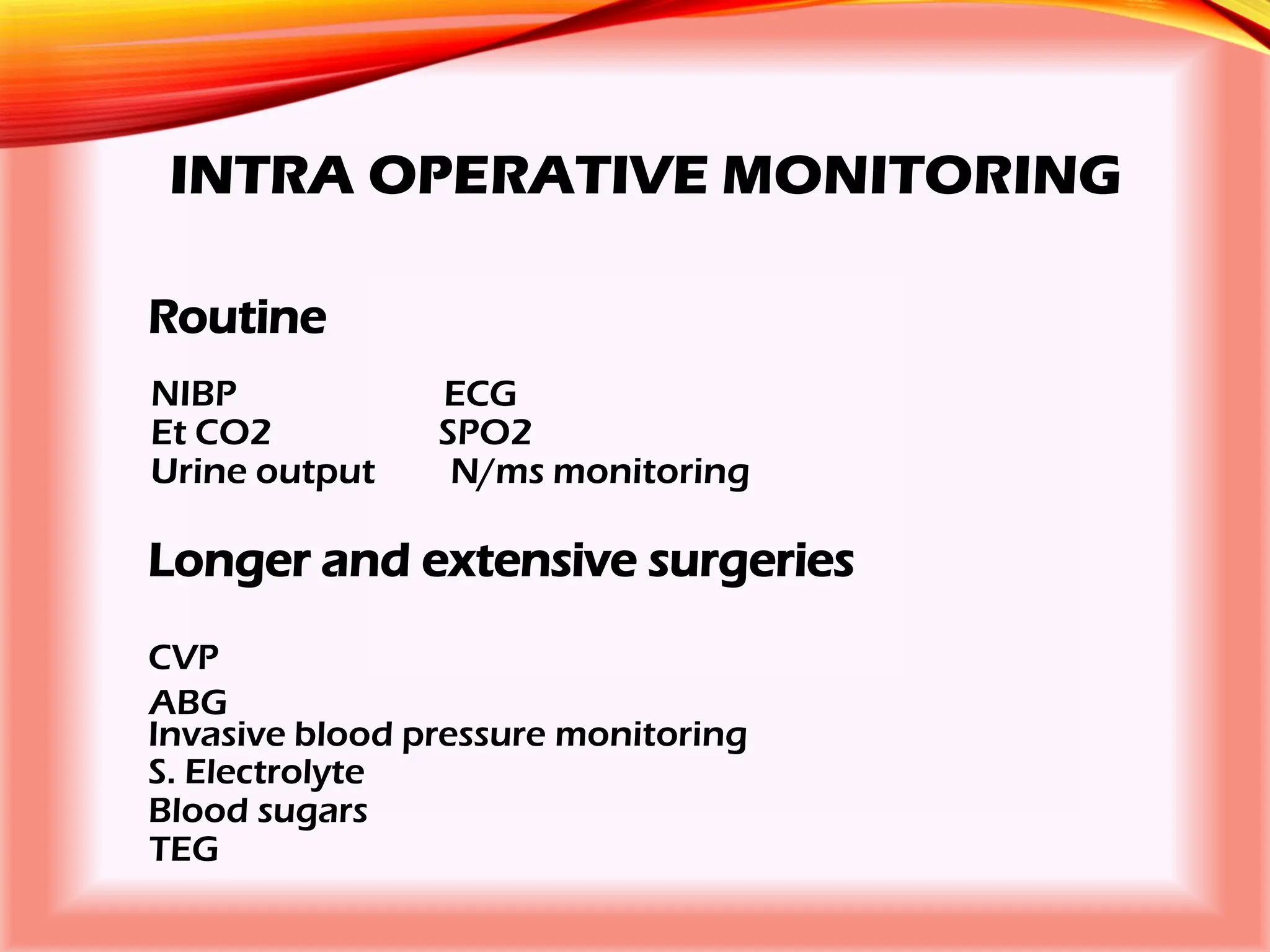
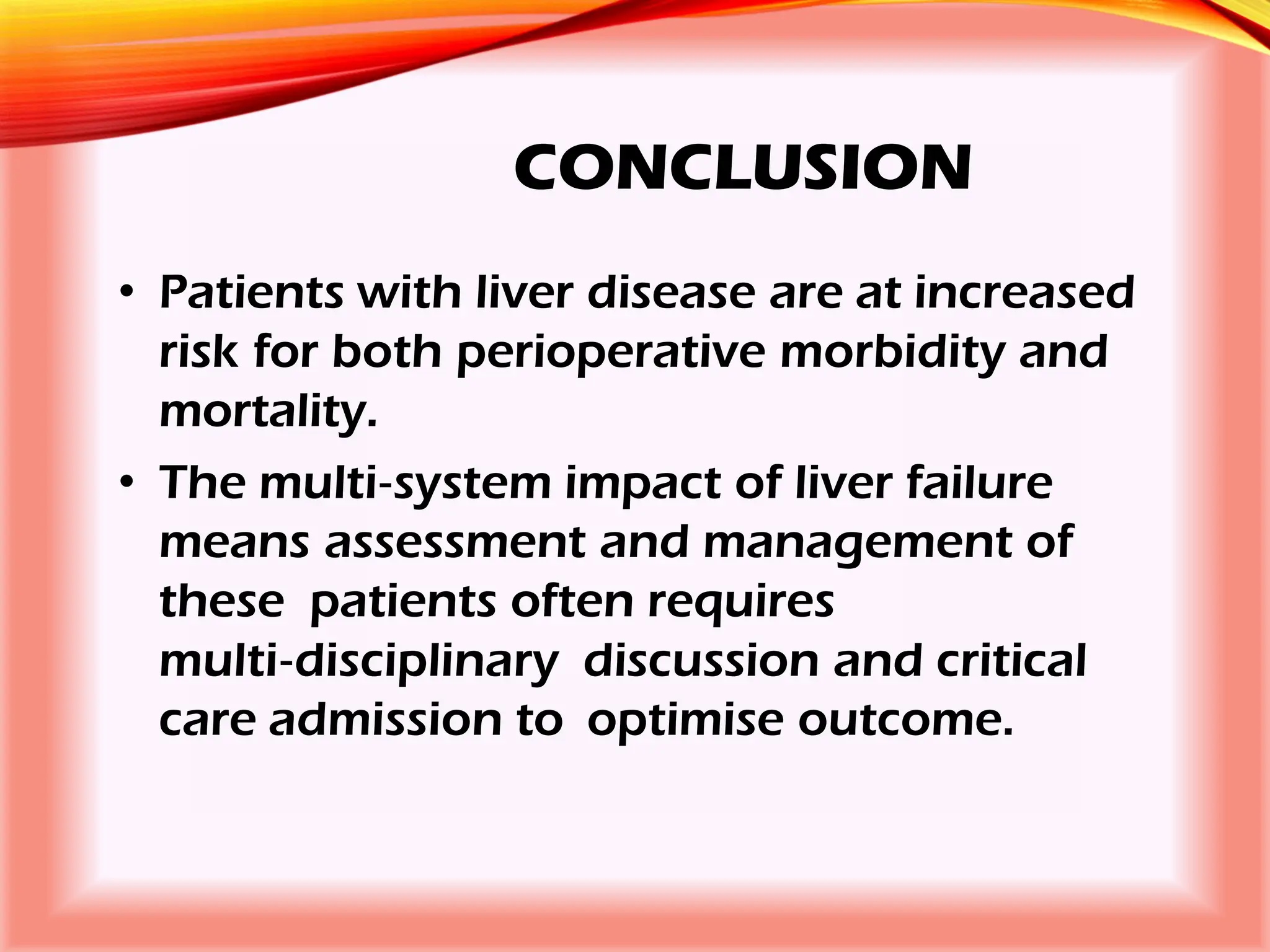
The document outlines the perioperative management of patients with liver disease, focusing on preoperative assessment, surgical risks, and anesthetic considerations. Key assessment includes evaluating liver function, cardiac status, and coagulopathy to inform surgical safety and postoperative care. It underscores the importance of multidisciplinary approaches to manage the complex risks associated with liver dysfunction during surgery.