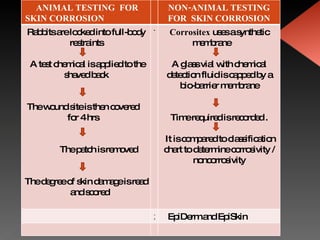
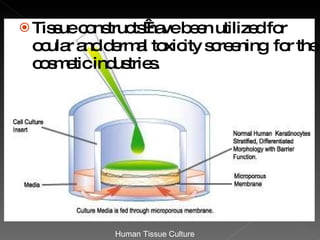
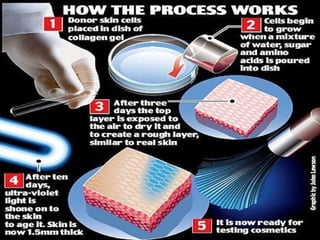
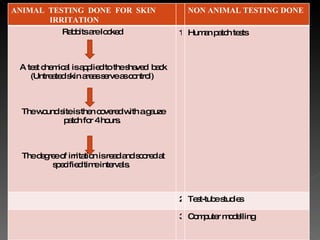
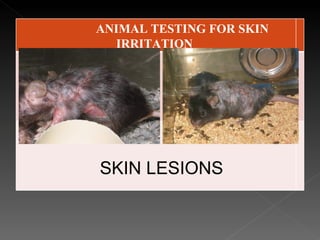
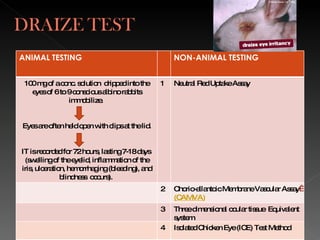
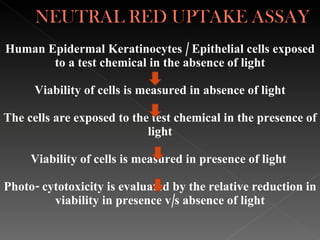
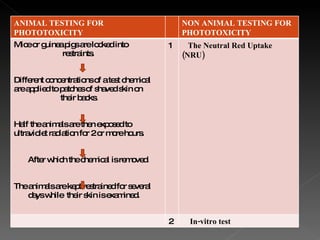
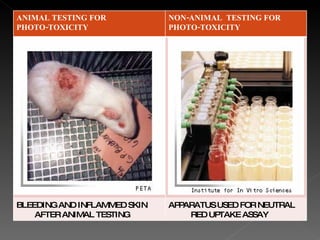
Animal testing is commonly used for safety testing but causes harm to animals. Non-animal testing methods provide alternatives like using synthetic membranes and human cell cultures to assess skin corrosion and absorption without harming animals. These alternative methods are becoming more widely used and accepted in the cosmetics industry for tests like skin irritation and phototoxicity testing.