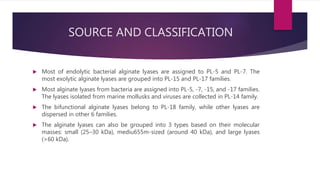
The document discusses the production and applications of alginate lyase, an enzyme that depolymerizes alginate into oligosaccharides, highlighting its fermentation process using Klebsiella pneumoniae with specific carbon sources. It details the enzyme's classification, mode of action, and its use in developing silver nanocomposites for treating Pseudomonas aeruginosa infections, showing promising results in eradicating the bacteria. Additionally, alginate lyase's potential in biocatalysis for the renewable sourcing of biochemicals and biofuels is outlined.