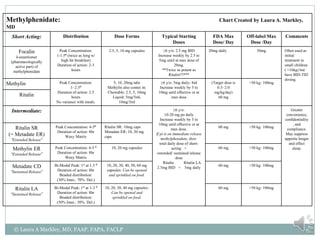
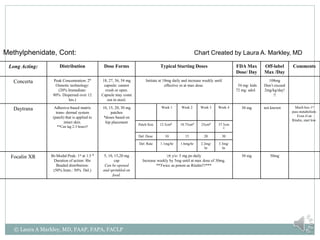
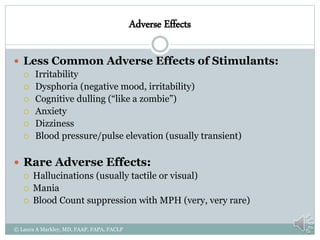
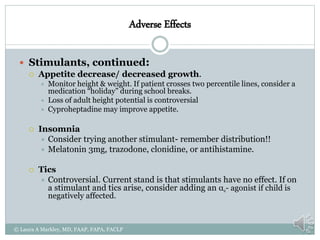
The document provides information on Attention Deficit Hyperactivity Disorder (ADHD) according to the DSM-V criteria. It summarizes the symptoms of inattention and hyperactivity/impulsivity required for a diagnosis. It also discusses diagnostic requirements such as the age of onset, presence of symptoms in multiple settings, and functional impairment. The document then summarizes treatment options for ADHD including stimulant medications, listing both short and long-acting options, and behavioral interventions. It provides dosage and safety information on the various medication options.















![Sources:
American Academy of Child and Adolescent Psychiatry. “Practice Parameter for the Assessment and Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity
Disorder. “Journal of the American Academy of Child and Adolescent Psychiatry. 2007; 46(7): 894-921.
American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. Journal of the
American Academy of Child and Adolescent Psychiatry. 2007;46(11):1503-1526.
American Academy of Child and Adolescent Psychiatry. “Practice Parameter for the Use of Psychotropic Medication in Children and Adolescents.” Journal of the American Academy of
Child and Adolescent Psychiatry. 2009; 48(9): 961-973.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association;
2000.
[An update on depression in children and adolescents]. Journal of Clinical Psychiatry. 2008;66(11):1818-1828.
Baer, Daniel. “Psychopharmacology Update.” 4th Ann. Development, Behavior and Emotions: Enhancing Care in the Medical Home. 4/8/10
Bloch, Michael H., M.D., et. al., “Meta-Analysis: Treatment of Attention-Deficit/ Hyperactivity Disorder in Children with Comorbid Tic Disorders.” Journal of the American Academy of
Child and Adolescent Psychiatry. 2009; 48(9): 884-893.
Boyer, et al. “Anticholinergic prophylaxis of acute haloperidol-induced acute dystonic reactions.” J Clin Psychopharmacol. 1987 Jun;7(3):164-6.
Caccia, Silvio. “Safety and Pharmacokinetics of Atypical Antipsychotics in Children and Adolescents” Pediatr Drugs (2013) 15:217–233
Callor, WB1, et al. “Preliminary findings of noncompliance with psychotropic medication and prevalence of methamphetamine intoxication associated with suicide completion.” Crisis.
2005;26(2):78-84.
Clonidine hydrochloride [Highlights of Prescribing Information]. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT; October 2009.
Daytrana™ (methylphenidate transdermal system) [Highlights of Prescribing Information]. Shire US Inc., Wayne, PA; December 2009.
Dittman, et al. “Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and co-morbid oppositional defiant disorder: a double-blind,
randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol. 2011 Apr;21(2):97-110. Epub 2011 Apr 13.
Emslie G, Kratochvil C, Vitiello B, Silva S, Mayes T, McNulty S, Weller E, Waslick B, Casat C, Walkup J, Pathak S, Rohde P, Posner K, March J. Treatment of adolescents with depression
study (TADS): Safety results. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(12):1440-1455.
Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J. A double-blind, randomized, placebo-controlled study of fluoxetine in depressed children and
adolescents. Archives of General Psychiatry. 1997;54:1031-1037.
Emslie GJ, Ryan ND, Wagner KD. Journal of Clinical Psychiatry. 2005;66(Suppl 7):14-20.
© Laura A Markley, MD, FAAP, FAPA, FACLP](https://image.slidesharecdn.com/redovianadhd-210515034506/85/ADHD-Presenter-Michael-Redovian-MD-26-320.jpg)
![Sources:
Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Archives of General Psychiatry. 2005;62:165-172.
Faraone, Stephen, PhD; Biederman, Joseph, M.D, et. al. “Effects of Stimulants on Height and Weight: A Review of the Literature.” Journal of the American Academy of Child and Adolescent
Psychiatry. 2008; 47(9): 994-1009.
Fluoxetine [FULL PRESCRIBING INFORMATION]. Edgemont Pharmaceuticals, LLC Austin, TX 78731; October 2011.
FOCALIN XR (dexmethylphenidate hydrochloride) [Highlights of Prescribing Information]. Novartis Pharmaceuticals Corporation, East Hanover, New Jersey; October 2009.
Gleason, Mary Margaret, M.D., et. al. “Psychopharmacological Treatment for Very Young Children: Contexts and Guidelines.” Journal of the American Academy of Child and Adolescent
Psychiatry. 2007; 46(12): 1532-1546.
Green, Wayne Hugo. Child & Adolescent Clinical Psychopharmacology, 4th Ed. Philadelphia, Lippincott Williams & Wilkins, 2007.
Hammad TA, Laughren, T, Racoosin, J. Suicidality in pediatric patients treated with antidepressant drugs. Archives of General Psychiatry. 2006;63:332-339.
Hilt, RJ. “Monitoring psychiatric medications in children” Pediatric Ann. 2012 Apr;41(4):157-63
Imipramine HCl Oral [Highlights of Prescribing Information]. Mallinckrodt Inc. Hazelwood, MO; August 2007.
INTUNIV™ (guanfacine) [Highlights of Prescribing Information]. Shire US Inc., Wayne, PA; August 2009.
Jain, R., et al, Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. Am Acad Child Adolesc Psychiatry. 2011 Feb;50(2):171-9
Jain, R., et al. “Clonidine Extended-Release Tablets as Add-on Therapy to Psychostimulants in Children and Adolescents With ADHD.” PEDIATRICS Volume 127, Number 6, June 2011
Kapvay® (clonidine hydrochloride) [Highlights of Prescribing Information] Shionogi Pharma, Inc., Atlanta, GA 30328; September, 2010.
Kratochchvil, Christopher J., M.D. “ADHD: Evidence-Based Treatments and Clinical Challenges.” AACAP 2009 Annual Meeting.
Kratochvil C, Emslie G, Silva S, McNulty S, Walkup J, Curry J, Reinecke M, Vitiello B, Rohde P, Feeny N, Casat C, Pathak S, Weller E, May D, Mayes T, Robins M, March J. Acute time to
response in the Treatment for Adolescents With Depression Study (TADS). Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1412-1418.
Libby AM, Brent DA, Morrato EH, Orton HD, Allen R, Valuck RJ. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. American Journal of
Psychiatry. 2007;164(6):884-91.
Libby AM, Orton HD, Valuck RJ. Persisting decline in depression treatment after FDA warnings. Archives of General Psychiatry. 2009;66(6):633-9.
Marwick, et al. “Antipsychotics and abnormal liver function tests: systematic review.” Clin Neuropharmacol. 2012 Sep-Oct; 35(5):244-53.
© Laura A Markley, MD, FAAP, FAPA, FACLP](https://image.slidesharecdn.com/redovianadhd-210515034506/85/ADHD-Presenter-Michael-Redovian-MD-27-320.jpg)
![Sources:
Metadate CD® (methylphenidate HCl, USP) [Highlights of Prescribing Information]. UCB, Inc., Smyrna, GA; February, 2007.
Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Archives of General Psychiatry. 2003;60:978-982.
Pamelor® (nortriptyline HCl) [Highlights of Prescribing Information]. Novartis Consumer Health, Inc., Lincoln, Nebraska; July 2006.
Perrin, J.M.; Friedman, Richard A.; Knilans, Timothy K., the Black Box Warning Group, and the Section on Cardiology and Cardiac Surgery. “Cardiovascular Monitoring and Stimulant Drugs
for Attention-Deficit/Hyperactivity Disorder.” Pediatrics. 2008; 122 (2): 451-453.
Pliszka, Stephen, M.D., and the CMAP (Children’s Medication Algorithm Project. “CMAP: Attention-Deficit/Hyperactivity DisorderAlgorithms.”
http://www.dshs.state.tx.us/mhprograms/adhdpage.shtm. Last updated 2006
Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. “Randomized, Controlled, Crossover Trial of Methylphenidate in Pervasive Developmental disorders with
hyperactivity.” Arch Gen Psychiatry. 2005; 62: 1266-1274.
Ritalin LA® (methylphenidate hydrochloride) [Highlights of Prescribing Information]. Novartis Pharmaceuticals Corporation, East Hanover, New Jersey; April 2009.
Ryan ND. Treatment of depression in children and adolescents. Lancet. 2005;366:933-940.
Sallee, Floyd R., M.D., et. al., “Long Term Safety and Efficacy of Guanfacine Extended Release in Children and Adolescents with Attention-Deficit/ Hyperactivity Disorder.” Journal of Child
and Adolescent Psychopharmacology. 2009; 19(3): 215-226.
Strattera [Highlights of Prescribing Information]. Shire US Inc., Wayne, PA; August 2009.
TADS. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled
trial. JAMA 2004;292:807-820
Tsapakis, EM, Soldani, F, Tondo, L, Baldessarini, RJ. Efficacy of antidepressants in juvenile depression: meta-analysis. The British Journal of Psychiatry. 2008;193:10-17.
Vyvanse® (lisdexamfetamine dimesylate) [Highlights of Prescribing Information]. Shire US Inc., Wayne, PA; December 2009.
Waknine,Yael. “FDA Approves Extended-Release Clonidine for Pediatric ADHD.” Medscape Medical News © 2010 WebMD, LLC
Wellbutrin [Highlights of Prescribing Information]. GlaxoSmithKline, Research Triangle Park, NC; July 2009.
Wellbutrin SR [Highlights of Prescribing Information]. GlaxoSmithKline, Research Triangle Park, NC; July 2009.
Wellbutrin XL [Highlights of Prescribing Information]. GlaxoSmithKline, Research Triangle Park, NC; December 2008.
Wiet, Susan, et. al.. Psychopharmacology Reference Table. Property of University of Utah Behavioral Health Clinic. Updated August 2008.
© Laura A Markley, MD, FAAP, FAPA, FACLP](https://image.slidesharecdn.com/redovianadhd-210515034506/85/ADHD-Presenter-Michael-Redovian-MD-28-320.jpg)