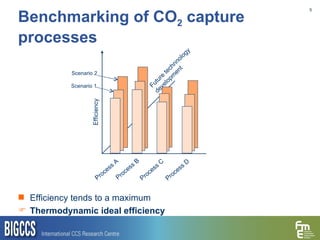
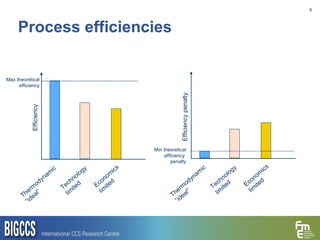
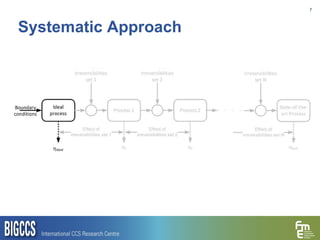
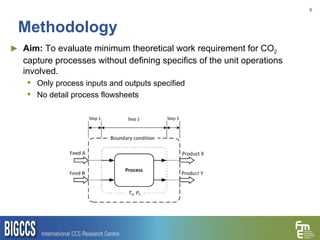
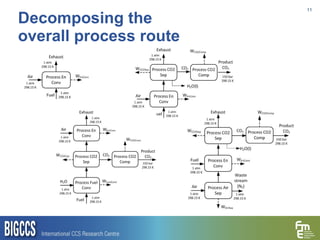
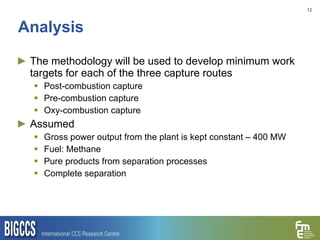
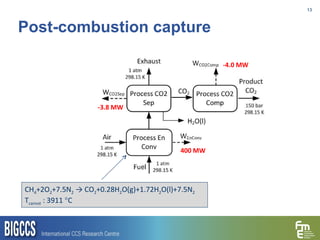
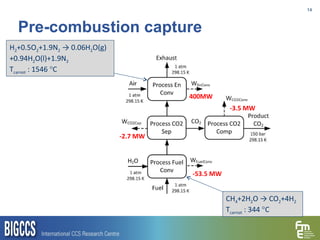
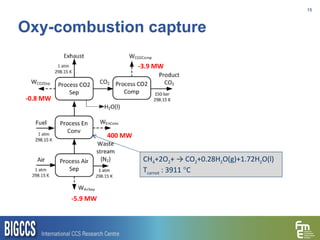
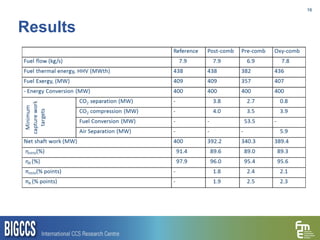
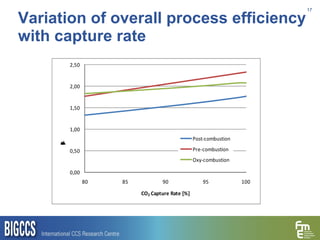
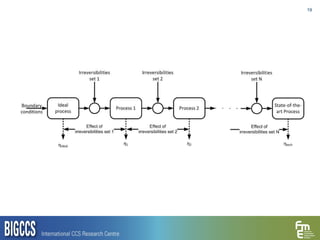
The document presents a methodology for benchmarking CO2 capture processes using minimum work targets. It decomposes CO2 capture processes into identifiable steps and calculates the minimum energy requirement for each step and the overall process. This provides an efficiency target for comparing different CO2 capture routes. Applying the methodology to post-combustion, pre-combustion and oxy-combustion capture routes shows that pre-combustion capture has the lowest minimum work requirement and highest efficiency. The methodology provides a basis for comparing processes and identifying areas for efficiency improvements.
![Benchmarking Methodology for CO 2 Capture Processes using Minimum Capture Work Targets Rahul Anantharaman , Kristin Jordal and David Berstad SINTEF Energy Research [email_address] Novi Sad, Serbia 06.07.2011](https://image.slidesharecdn.com/ecos2011abenchmarkingmethodologyforco2captureprocesses-110708022717-phpapp01/85/A-benchmarking-methodology-for-CO2-capture-processes-1-320.jpg)