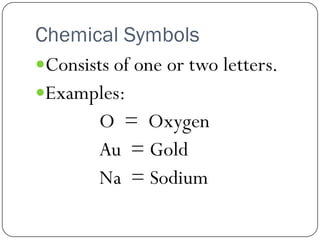
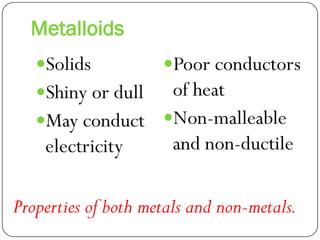
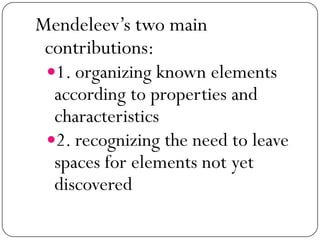
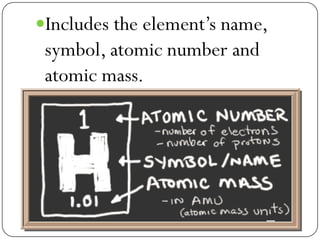
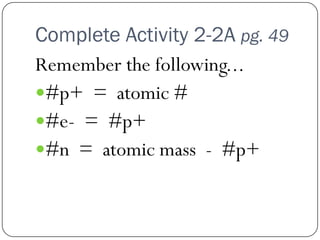
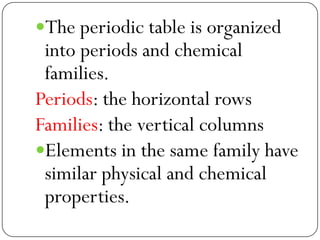
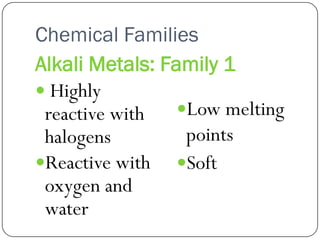
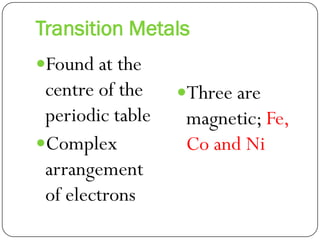
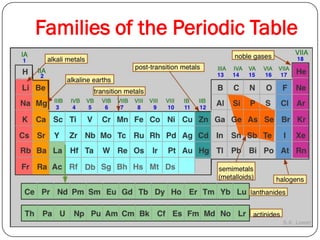
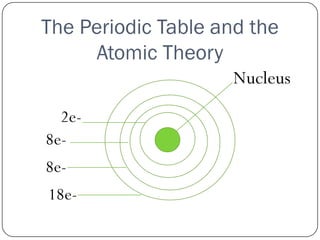
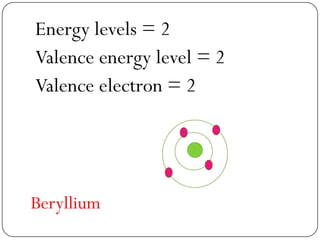
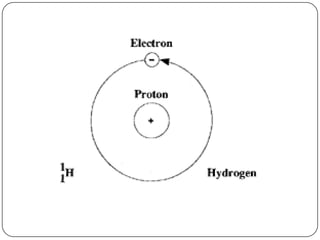
This document discusses the key elements chapter of a grade 9 science textbook. It defines elements as pure substances made of one type of atom that cannot be broken down further. There are over 115 known elements, each represented by a one or two letter chemical symbol like O for oxygen or Au for gold. Elements can be metals, non-metals, or metalloids, with different characteristic properties. The periodic table organizes all elements according to their atomic structure and was developed by Dmitri Mendeleev in 1867 to show relationships between elements. The periodic table includes each element's name, symbol, atomic number and mass.