Embed presentation
Download to read offline
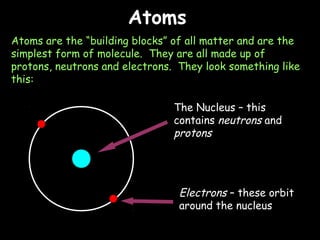


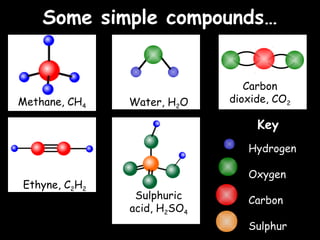


Atoms are the building blocks of matter and are composed of protons, neutrons and electrons. Elements are composed of only one type of atom, while compounds contain two or more different types of atoms bonded together. Some common elements include hydrogen, helium, carbon, oxygen, sodium, and iron. Compounds such as water, methane, glucose and sodium chloride are composed of different combinations of elements.






