This document provides information on the treatment of metastatic renal cell carcinoma. It discusses current targeted therapies for RCC including inhibitors of VEGF and mTOR pathways such as sunitinib, sorafenib, everolimus and temsirolimus. Patient outcomes with various first and second line targeted therapies are presented. Guidelines for cytoreductive nephrectomy and metastasectomy are also summarized.


























![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
• In the era of immunotherapy, cytoreductive nephrectomy was
recommended in patients with good PS [I, A].
• Whether this recommendation will remain with current targeted
therapies is currently being investigated in two prospective trials.
• In routine practice, cytoreductive nephrectomy is recommended
in patients with good PS and large primary tumours with limited
volumes of metastatic disease, and for patients with a
symptomatic primary lesion. Cytoreductive nephrectomy is not
recommended in patients with poor PS.
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-27-320.jpg)











































![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
• Bisphosphonate therapy with zoledronic acid has been
shown to reduce skeletal-related events in patients with
bone metastasis due to mRCC and they should be
considered for zoledronic acid treatment, weighting the
potential benefits of the treatment (supposed benefit in
terms of OS) with the potential harms (risk of
osteonecrosis of the jaw) [II, A].
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
1 Lipton A et al Zoledronic acid delays the onset of skeletal-related
events and progression of skeletal disease in patients with advanced renal cell
carcinoma. Cancer 2003.
2 Aapro M et al. Guidance on the use of
bisphosphonates in solid tumours: recommendations of an international expert
panel. Ann Oncol 2008.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-76-320.jpg)










![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
• Patients are stratified according to the presence of six
risk factors [International Metastatic RCC Database
Consortium (IMDC) criteria] 0, 1-2, 3-6:
• Karnofsky performance status (PS) <80%
• Haemoglobin <lower limit of normal
• Time from diagnosis to treatment of <1 year
• Corrected calcium above the upper limit of normal
• Platelets greater than the upper limit of normal
• Neutrophils greater than the upper limit of normal
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-88-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
first-line treatment of patients with good or intermediate
prognosis
• Because some RCC have a very indolent course, a period of
observation before starting treatment should be considered,
especially in patients with limited tumour burden and few
symptoms.
• 3 treatments have demonstrated efficacy in pivotal phase 3:
bevacizumab (IFN-α), sunitinib and pazopanib [I, A].
• Improvement of PFS over either IFN-α or placebo.
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
1 Escudier B et al. Bevacizumab plus interferon alfa-2a
for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase
III trial. Lancet 2007.
2 Motzer R et al. Sunitinib versus interferon alfa in
metastatic renal-cell carcinoma. N Engl J Med 2007.
3 Sternberg CN et al. Pazopanib in locally advanced or
metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol
2010.
4 Motzer RJ et al. Pazopanib versus sunitinib in metastatic renalcell
carcinoma. N Engl J Med 2013.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-89-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
first-line treatment of patients with good or
intermediate prognosis
• Sorafenib [II, B], high-dose interleukin-2 [III, C] and
low-dose IFN-α combined with bevacizumab [III, A]
are options.
• Single agent IFN-α, the losing arm of three
randomised, controlled trials, should no longer be
regarded as a standard option [I, D].
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-90-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
first-line treatment of patients with poor prognosis
• Temsirolimus is currently the only drug with level I evidence of
activity in this patient population [II, A]. The pivotal trial
demonstrated improvement of OS compared with IFN-α or
combination of temsirolimus and IFN-α.
• Based on subgroup analysis from the pivotal trial as well as
expanded access programmes, sunitinib is another reasonable
option in this setting [II, B]. Sorafenib based on expanded access
programmes is another possible alternative [III, B]. It is clear that,
for some poor prognosis patients, best supportive care remains
the only suitable treatment option.
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
Hudes G et al. Temsirolimus, interferon alfa, or both for
advanced renal-cell carcinoma. N Engl J Med 2007.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-91-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
second-line treatment
• Evidence that TKIs are active after cytokines has been
demonstrated with sorafenib [I, A], pazopanib [II, A] and
recently axitinib [I, A]. Sunitinib also has activity in this
setting [III, A].
• However, since VEGF-targeted therapy is now the first-
line standard of care, the number of patients treated
with cytokines is decreasing.
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
1 Sternberg CN et al. Pazopanib in locally advanced or
metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol
2010.
2 Escudier B et al. Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med 2007.
3 Rini BI et al. Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial.
Lancet 2011.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-92-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
second-line treatment
• After first-line treatment with VEGF-targeted therapy
○ Both axitinib [I, B] and everolimus [II, A] are active. Both
drugs have shown significantly improved PFS over
placebo (everolimus) or sorafenib (axitinib), but not OS.
○ Based on recent phase III trials, sorafenib can be used as
an option [II, A].
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
1 Rini BI et al. Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial.
Lancet 2011.
2 Motzer RJ et al. Efficacy of everolimus in advanced renal
cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial.
Lancet 2008.
3 Hutson TE et al. Randomized phase III trial of temsirolimus
versus sorafenib as second-line therapy after sunitinib in patients with metastatic
renal cell carcinoma. J Clin Oncol 2014.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-93-320.jpg)
![Metastatic Renal Cell Carcinoma:
ESMO Clinical Practice Guidelines
third-line treatment
• Beyond second-line treatment, enrolment into clinical trials is
recommended where possible. However, some recent trials have
been reported, helping to define two different scenarios:
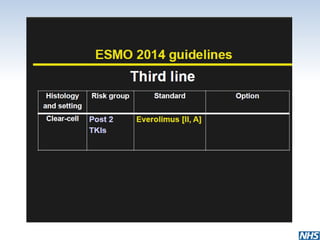
• In patients already treated with two TKIs (or a TKI and
bevacizumab), everolimus is recommended [II, A].
• In patients previously treated with VEGF-targeted
therapy and mTOR inhibitor, sorafenib [I, B] has shown
activity. Another TKI or rechallenge with the same TKI is
considered as an option [IV, B].
Annals of Oncology 25 (Supplement 3): iii49–iii56, 2014
Motzer RJ et al. Dovitinib versus sorafenib for third-line
targeted treatment of patients with metastatic renal cell carcinoma: an open-label,
randomised phase 3 trial. Lancet Oncol 2014.](https://image.slidesharecdn.com/tsoukalasnrenalcancerrodos2016finalok-160530075726/85/4-94-320.jpg)