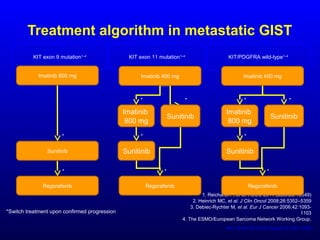
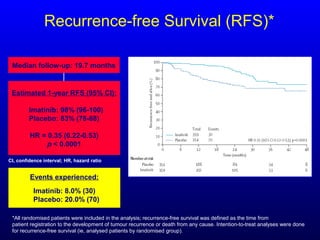
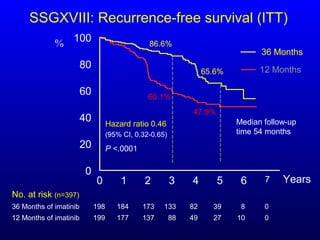
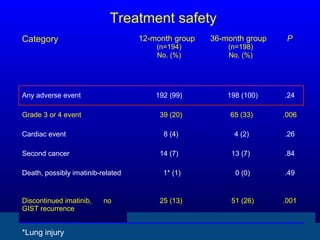
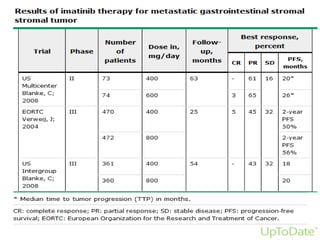
This document discusses gastrointestinal stromal tumors (GIST) and the use of adjuvant imatinib therapy. It provides background on GIST epidemiology and risk factors for recurrence. Studies show adjuvant imatinib for 1 year after surgery significantly reduces recurrence rates compared to placebo, especially for high-risk patients. The SSGXVIII trial found adjuvant imatinib for 3 years further reduced recurrence rates compared to 1 year of treatment, with no significant difference in overall survival yet. Adjuvant imatinib for 3 years may provide additional benefit over 1 year, especially for high-risk GIST patients.








![Imatinib: Selective TKI Targeting KIT,
PDGFRA, and Abl
Approved for treatment of
unresectable, advanced
KIT-positive GIST[33,34]
and
as adjuvant therapy for
resectable GIST[35]
32. Rubin BP, et al. Lancet. 2007;369:1731-1741. 33. Demetri GD, et al. N Engl J Med. 2002;347:472-480.
34. Blanke CD, et al. J Clin Oncol. 2008;26:626-632. 35. DeMatteo RP, et al. Lancet. 2009;373:1097-1104.
Mechanism of action: Imatinib binds to the same site as ATP, thereby preventing
phosphorylation of downstream substrates and inhibiting KIT or PDGFRA signaling[32]
Imatinib Mesylate
N
N
N
H
N
H
N
N
N
O CH3So3H
Inhibition of KIT activated signal
transduction, causing reduced GIST
proliferation or induction of apoptosis
KIT-activated signal transduction
resulting in GIST proliferation
and survival
P
P
ADP P Y Substrate
ADP
P
P
P
IMATY SubstrateADPADP
P
P
P
P
P
P
A B
IMAT
ADP
P
P
P
Y Substrate](https://image.slidesharecdn.com/strimpakosgist-160530082434/85/4-11-320.jpg)





















![Regorafenib: Novel Multitargeted TKI
Regorafenib has a wide spectrum of
target inhibition: KIT; PDGFR; VEGFR-1,
-2, -3; TIE2; RET, fibroblast growth factor
receptor 1; RAF; and p38 MAPK[63]
Phase II study (N = 33) in metastatic
GIST: 4 PRs, 22 SD ≥ 22 wks, median
PFS: 10 mos[63]
Phase III GRID study[64]
– Significant PFS improvement vs
placebo in 199 pts with metastatic
or unresectable GIST and
progression on imatinib and
sunitinib
– Most common grade 3/4 events:
hand-foot skin reaction, hypertension,
diarrhea
63. George S, et al. J Clin Oncol. 2012;30:2401-2407.
64. Demetri G, et al. ASCO 2012. Abstract LBA10008.
ProportionWithoutProgression
1.00
0.75
0.50
0.25
0
0 50 100 150 200 250 300
Days From Randomization
Placebo
Regorafenib
HR: 0.27 (95% CI: 0.19-0.39)
1-sided P < .0001
Regorafenib
(n = 133)
Placebo
(n = 66)
Median PFS,
mos (95% CI)
4.8
(4.1-5.8)
0.9
(0.9-1.1)
Events, n (%) 81 (60.9) 63 (95.5)](https://image.slidesharecdn.com/strimpakosgist-160530082434/85/4-38-320.jpg)