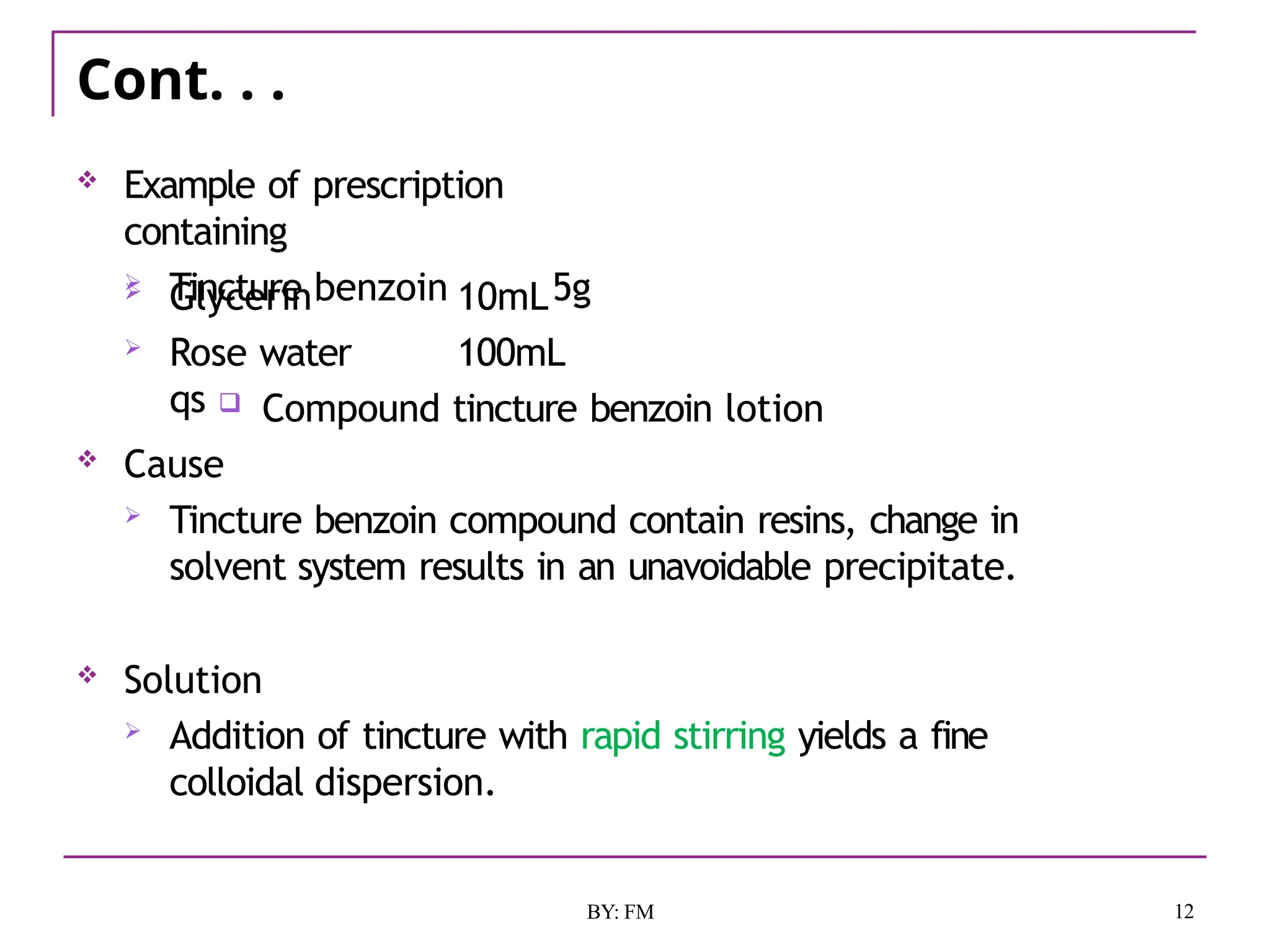
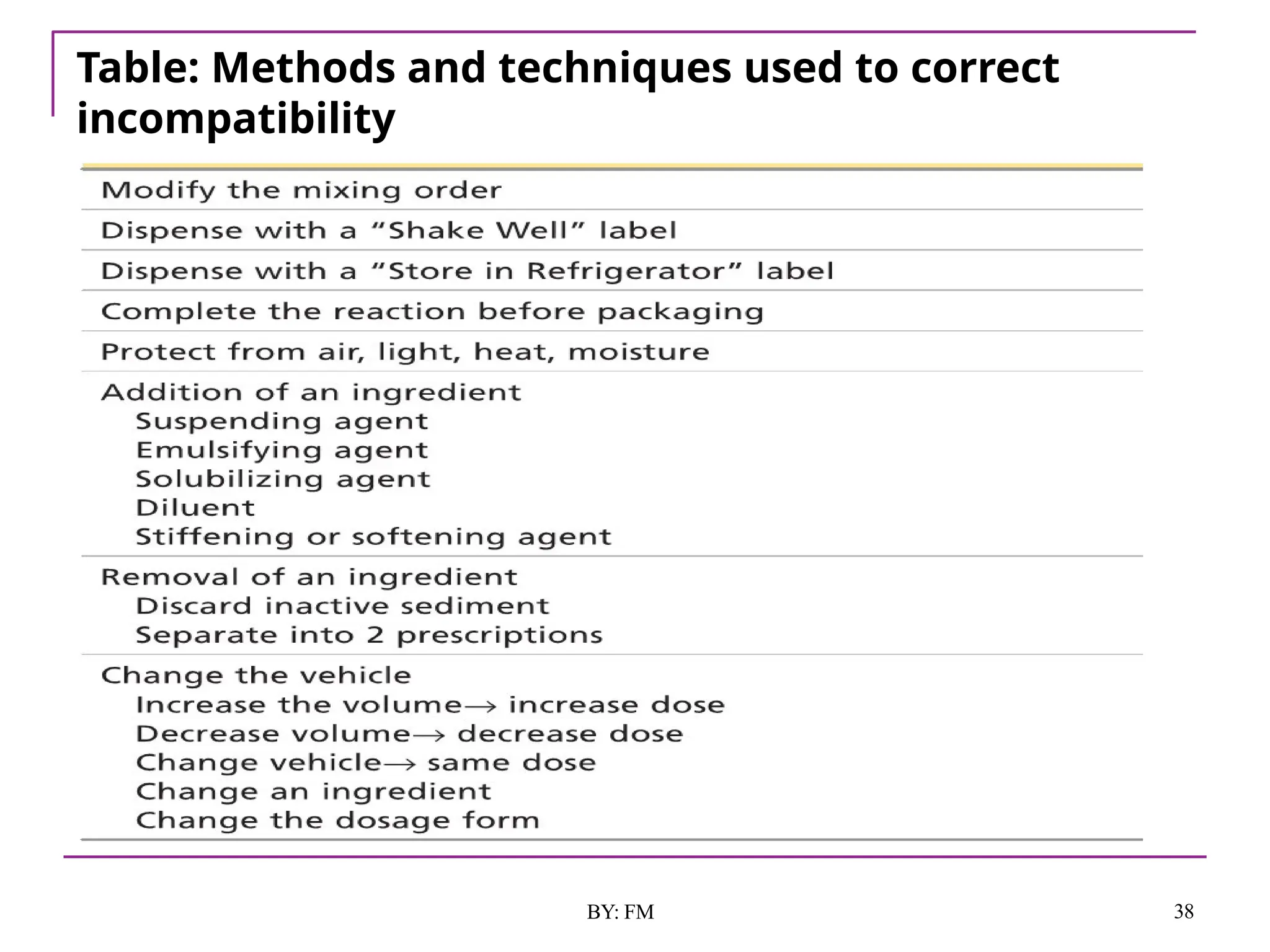
The document discusses the various types of incompatibilities in pharmaceutical formulations, including physical, chemical, and therapeutic incompatibilities. It explores definitions, manifestations, and correction methods for each type, providing examples and factors that lead to incompatibilities, such as changes in solubility and drug interactions. The importance of recognizing and correcting potential incompatibilities during drug formulation to ensure safety and efficacy is emphasized.