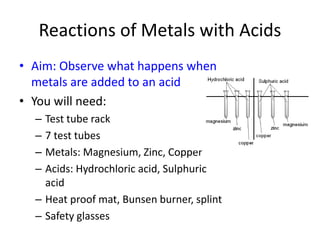
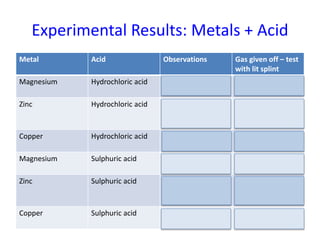
This document discusses reactions between metals and acids. When metals react with water, they produce metal hydroxides and hydrogen gas. Metals react more vigorously with acids, producing salts and releasing hydrogen gas. An experiment was conducted with magnesium, zinc, and copper reacting with hydrochloric and sulfuric acids. Magnesium and zinc reacted to produce magnesium chloride, zinc chloride, magnesium sulfate, and zinc sulfate, along with hydrogen gas. Copper did not react. The reactivity series of metals was updated based on these results.