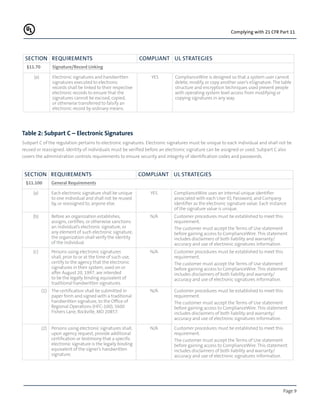
The document discusses the FDA's 21 CFR Part 11 regulation on electronic records and signatures. It provides definitions and terminology used in the regulation. The regulation requires three levels of control - administrative, procedural, and technical. It also contains two major subparts on electronic records and electronic signatures. Tables 1 and 2 show how the ComplianceWire system addresses the specific requirements for electronic records and signatures outlined in the two subparts. The purpose is to help FDA-regulated industries quickly and cost-effectively comply with 21 CFR Part 11.