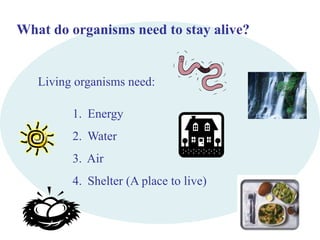
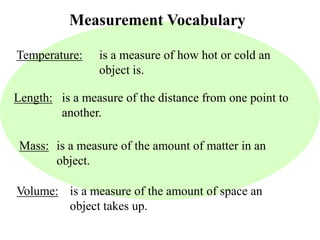
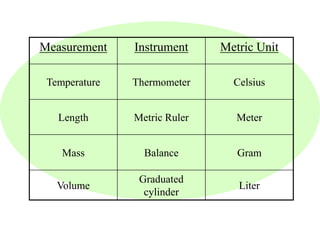
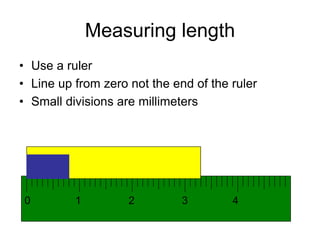
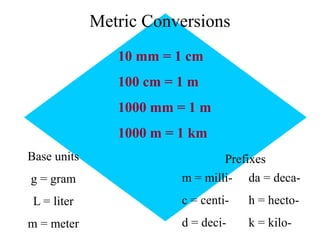
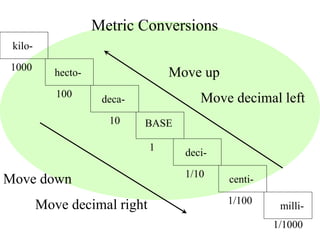
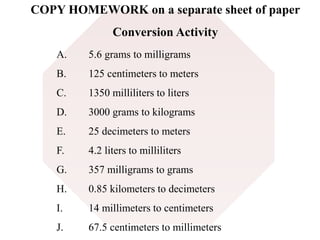
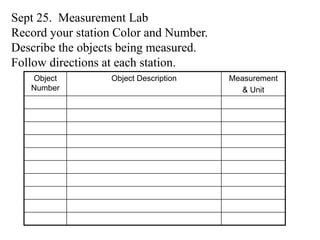
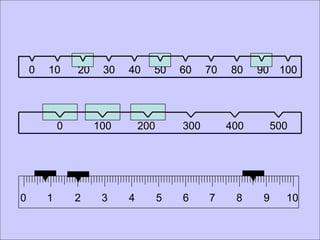
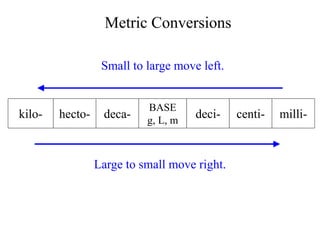
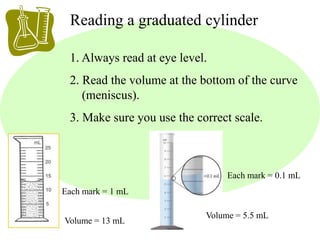
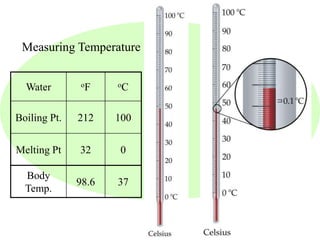
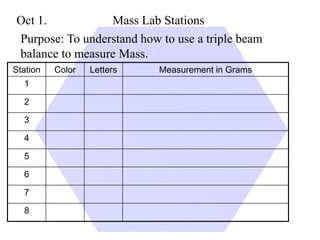
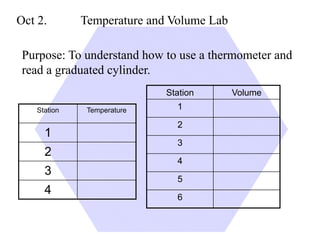
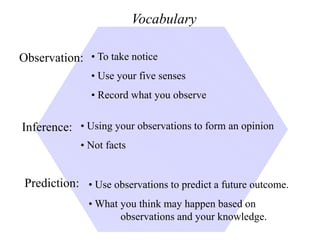
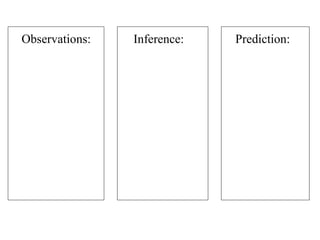
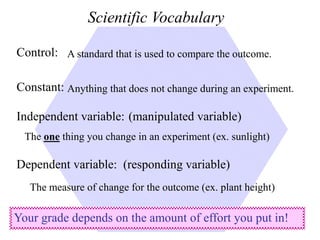
This document provides an overview of a science unit on living vs. non-living things and measurement. It includes objectives, activities, and instructions for students. They will identify characteristics of living things, needs for survival, complete a poster comparing living and non-living objects, and learn to use tools like thermometers and graduated cylinders to take measurements in metrics. Students are introduced to key scientific concepts like observations, inferences, predictions, and the scientific method to design experiments and analyze data. Safety procedures are also outlined for laboratory work.