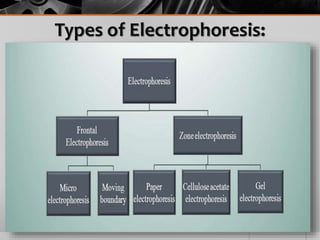
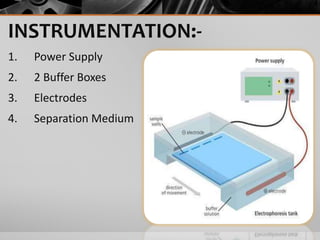
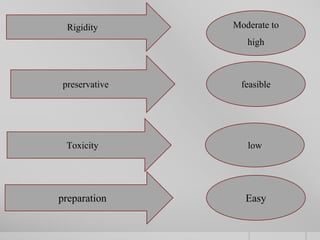
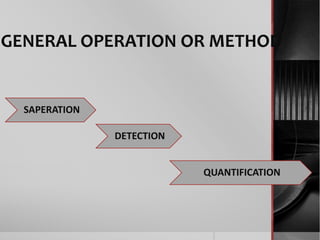
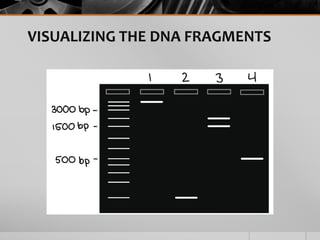
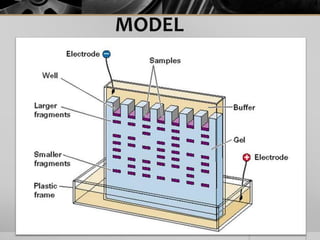
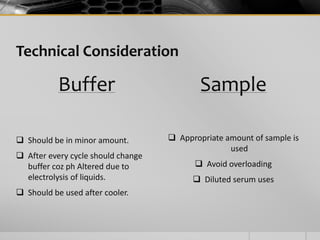
The document outlines the concept of electrophoresis, a technique for migrating charged particles in an electric field, highlighting its applications such as analyzing proteins and DNA. It discusses factors affecting electrophoretic mobility, instrumentation needed, and specific methods like gel electrophoresis. Clinical applications and important operational considerations for conducting electrophoresis are also summarized.