This document provides an overview of chemistry concepts covered in an AP Biology course. It discusses the following key points:
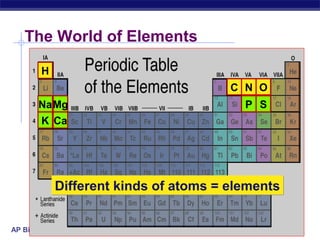
1) All matter is made of atoms, which are made of protons, neutrons, and electrons. The number of each defines the element.
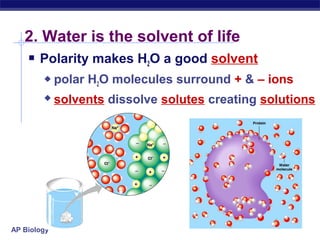
2) Life requires about 25 essential chemical elements, with carbon, hydrogen, oxygen, and nitrogen making up 96% of living matter.
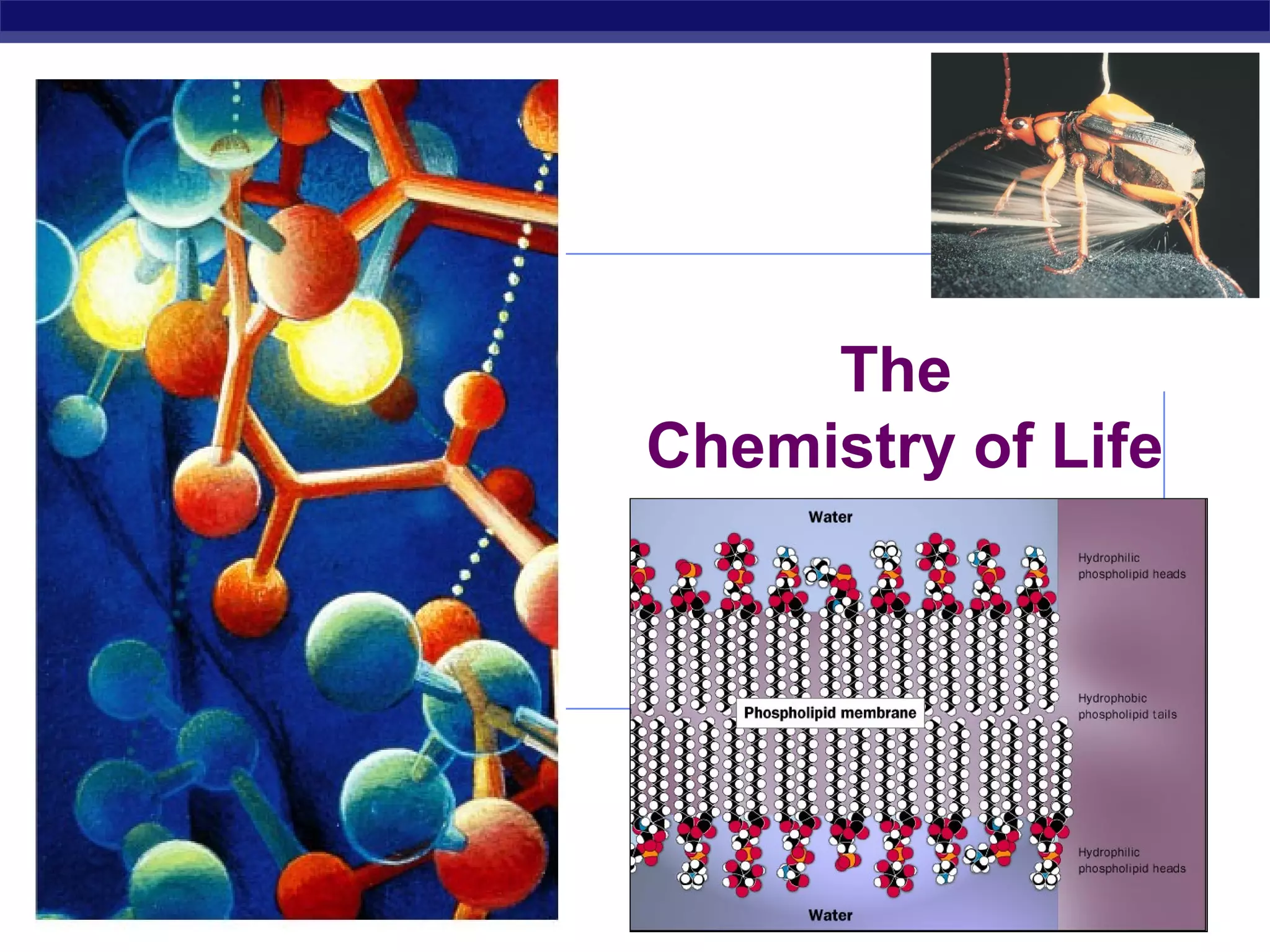
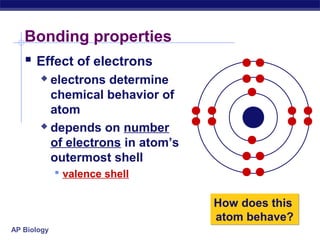
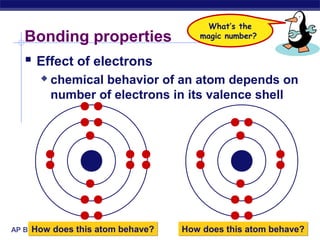
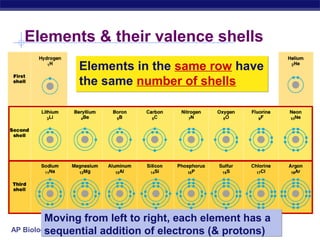
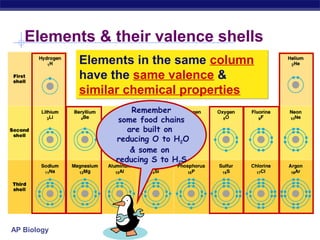
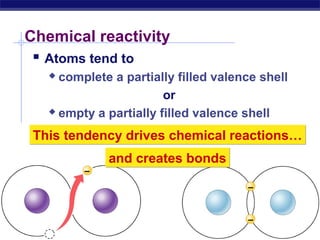
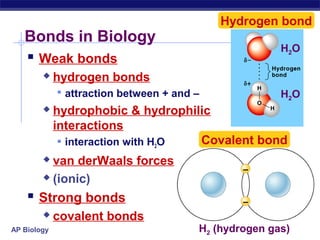
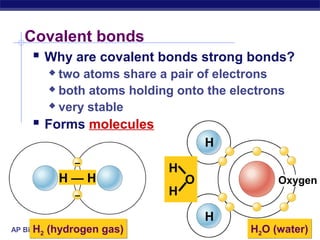
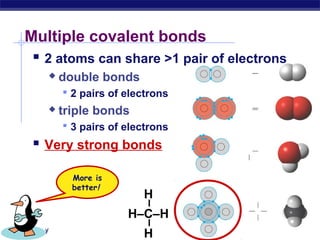
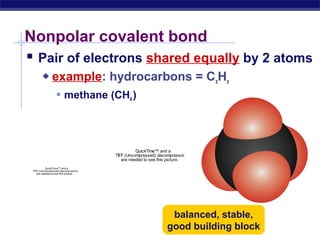
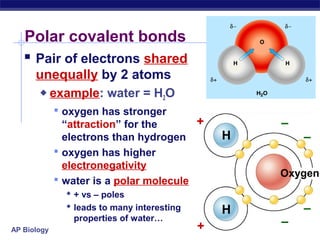
3) Atoms bond through sharing or transferring electrons to gain stable electron configurations. Covalent and ionic bonds are strong bonds important for molecules and biological structures.
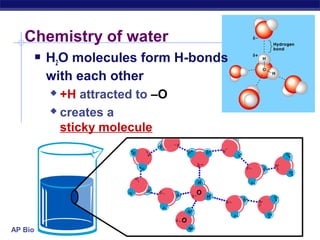
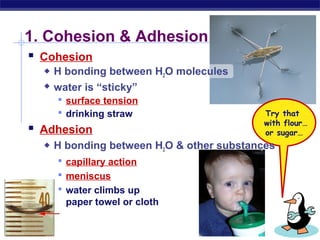
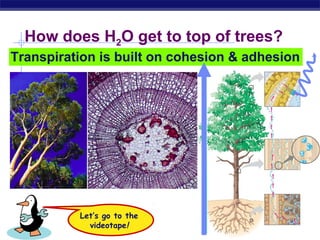
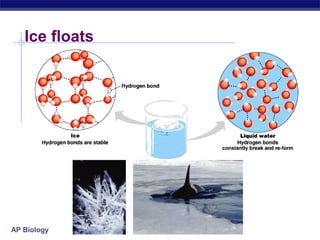
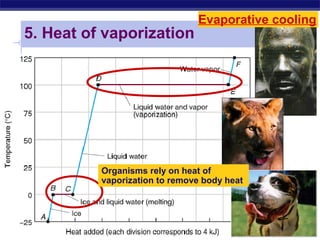
4) Water has unique properties like hydrogen bonding that allow it to act as the solvent for life and help regulate temperatures on Earth. Its heat










![AP Biology
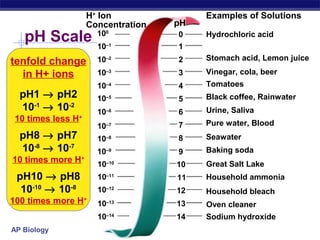
Ionization of water & pH
Water ionizes
H+
splits off from H2O, leaving OH–
if [H+
] = [-
OH], water is neutral
if [H+
] > [-
OH], water is acidic
if [H+
] < [-
OH], water is basic
pH scale
how acid or basic solution is
1 → 7 → 14 H2O → H+
+ OH–H2O → H+
+ OH–](https://image.slidesharecdn.com/20ch02chemistry2008-130917125427-phpapp02/85/20-ch02chemistry2008-32-320.jpg)
![AP Biology 10
0
1
2
3
4
5
6
7
8
9
3
Amount of base added
Buffering
range
4 52
pH
Buffers & cellular regulation
pH of cells must be kept ~7
pH affects shape of molecules
shape of molecules affect function
pH affects cellular function
Control pH by buffers
reservoir of H+
donate H+ when
[H+
] falls
absorb H+ when
[H+
] rises](https://image.slidesharecdn.com/20ch02chemistry2008-130917125427-phpapp02/85/20-ch02chemistry2008-34-320.jpg)

