This document provides an overview of key chemistry and biochemistry concepts relevant to microbiology. It discusses the structure and function of atoms, molecules, and chemical bonds including covalent bonds, ionic bonds, hydrogen bonds, and hydrophobic interactions. It also examines biologically important elements and how water interacts with hydrophilic and hydrophobic molecules. Additionally, it covers acid-base balance, properties of water, and the role of buffers in maintaining pH.






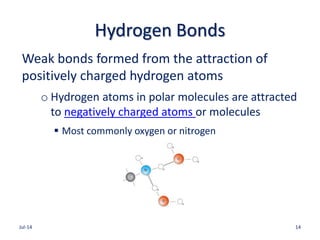



![Acid-Base Balance
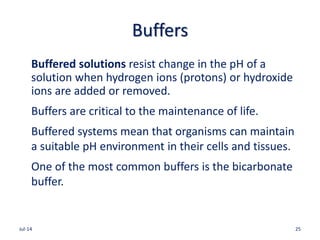
pH is a measure of the concentration of H+ in a
solution.
pH =7.0 is neutral (pH of freshly distilled water)
pH>7.0 is basic
pH<7.0 is acidic
pH = log 1/[H+]=log[H+]
Jul-14 21](https://image.slidesharecdn.com/2-140706220711-phpapp02/85/Chemistry-Review-for-Microbiology-Students-21-320.jpg)
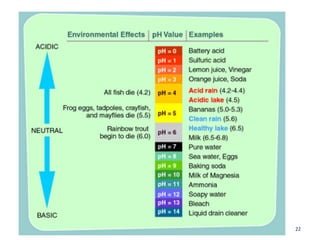
![Acid-Base Balance
Increasing [H+] increases acidity. pH decreases.
Increasing [OH] or pH increases alkalinity.
Most organisms grow best between pH 6.5 and 8.5
Optimal pH for most bacterial growth is slightly basic.
Optimal pH for fungal growth is slightly acidic.](https://image.slidesharecdn.com/2-140706220711-phpapp02/85/Chemistry-Review-for-Microbiology-Students-23-320.jpg)