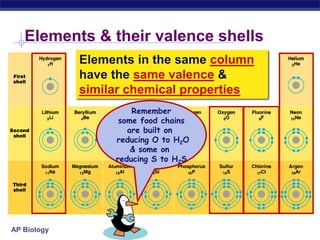
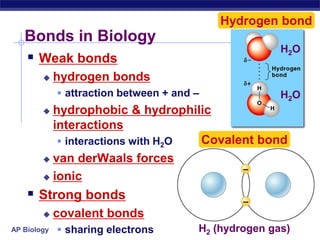
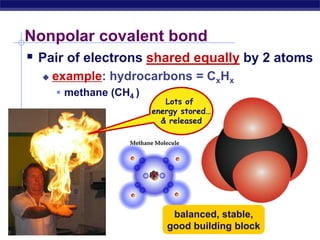
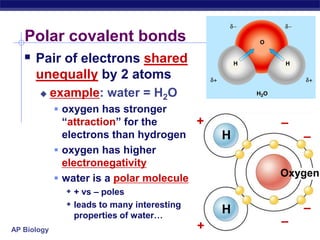
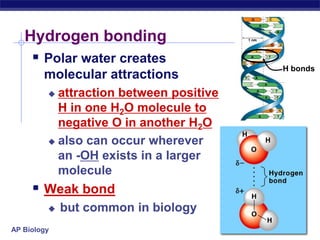
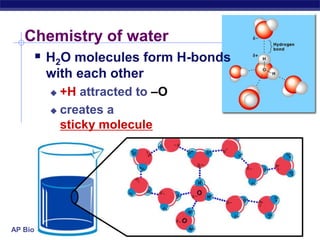
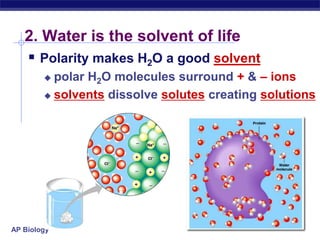
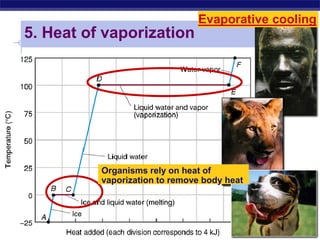
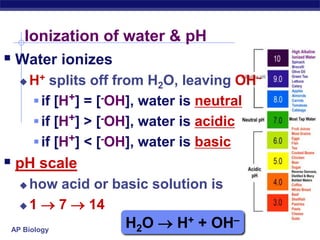
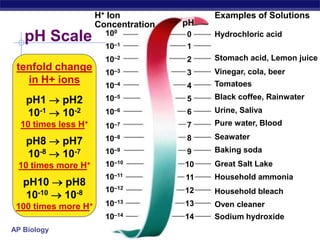
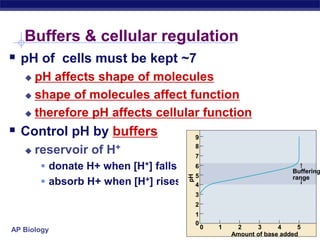
This document provides an overview of key chemistry concepts related to water and its importance for life. It discusses water's unique properties including its ability to form hydrogen bonds, act as a solvent, have lower density as a solid allowing ice to float, store and release heat via its high specific heat and heat of vaporization, and ionize into H+ and OH- ions determining pH. These special properties of water, especially its prevalence as the solvent for life processes and its role in moderating temperatures on Earth, make it uniquely suited to supporting life.