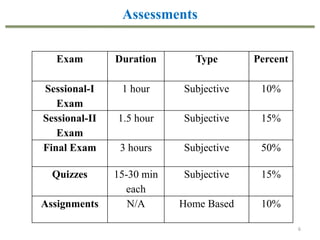
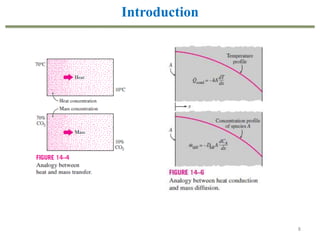
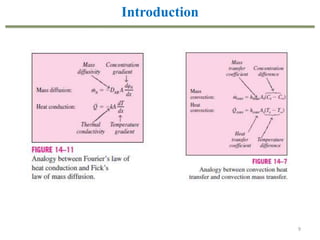
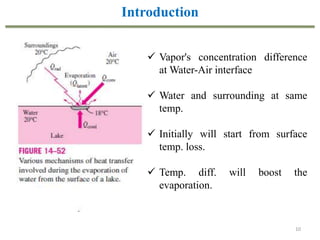
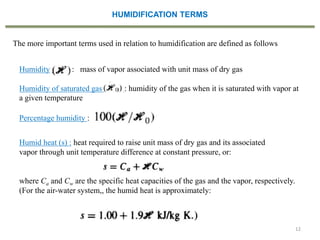
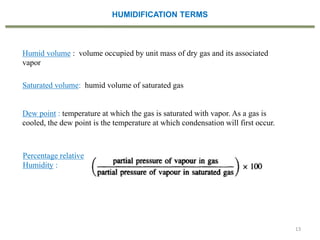
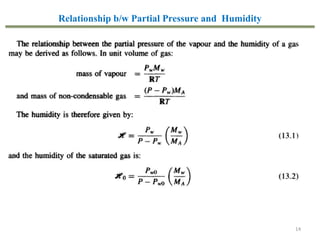
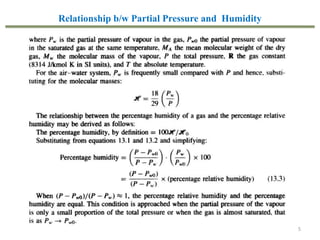
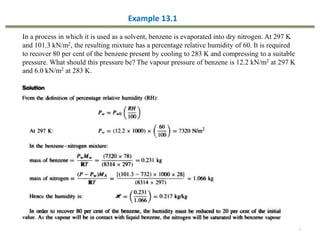
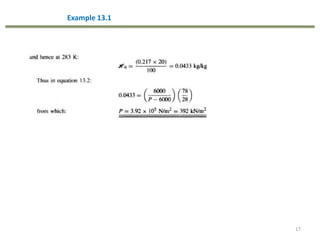
This document provides an overview of the CHE333 course on Simultaneous Heat and Mass Transfer Operations. It covers topics like humidification, cooling towers, drying, evaporation, distillation, and more. The course objectives are to elaborate on simultaneous heat and mass transfer phenomena for chemical engineering unit operations and apply these concepts to various equipment. Assessments include exams, quizzes, assignments, and presentations. Key terms related to humidification like humidity, humid heat, dew point, and relative humidity are also defined. Examples on humidification and the relationship between partial pressure and humidity are provided.