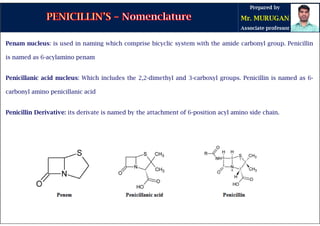
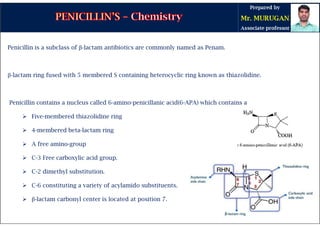
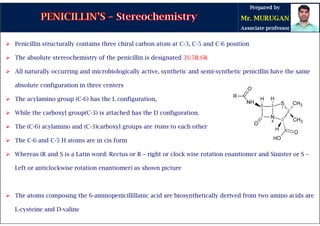
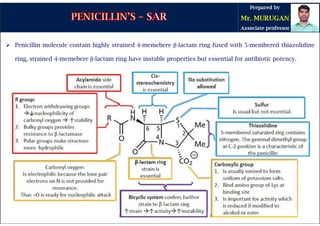
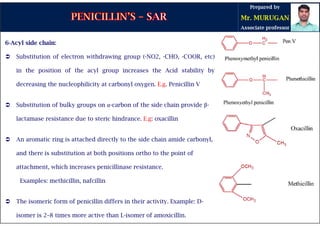
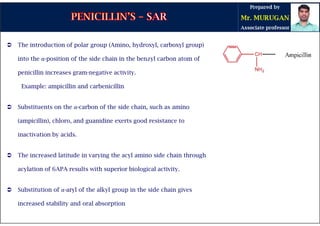
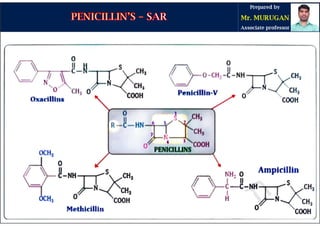
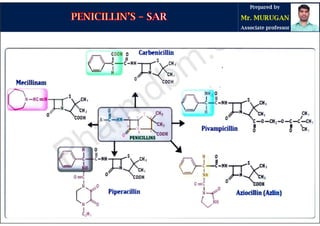
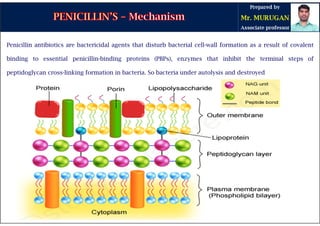
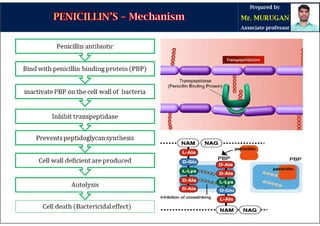
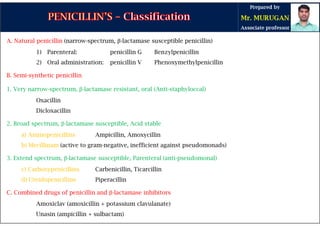
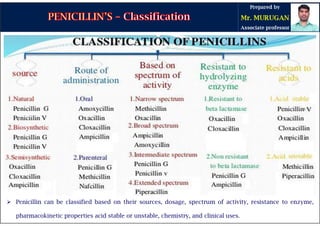
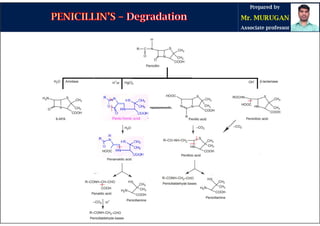
The document is a comprehensive overview of penicillins, detailing their historical context, nomenclature, chemical structure, mechanisms of action, classifications, and important derivatives. It emphasizes the development of antibiotic properties, including penicillin's origin, types, and uses in treating various infections, while also discussing degradation mechanisms and the differences between natural and semi-synthetic penicillins. Overall, it provides essential insights into the significance and application of penicillins in medicinal chemistry.


![•The nomenclature of penicillin is somewhat complex
•Two systems as follows. Chemical abstract system
United State Pharmacopeia system
•The Chemical Abstracts system initiates the numbering assigns with 1-
position sulfur atom and the ring nitrogen the 4-position. Thus, penicillin are
named as 4-thia-l-azabicyclo[3.2.0]heptane, according to this system.
•The numbering system adopted by the USP is the reverse of the Chemical
Abstracts procedure; which initiates the numbering assigns with 1-position
nitrogen atom and the ring Sulphur the 4-position. Thus, penicillin are named
as 1-thia-4-azabicyclo[3.2.0]heptane,](https://image.slidesharecdn.com/part3-penicillin-250101020147-3175af08/85/MC-III-Unit-1-Part-3-Penicillin-pdf-5-320.jpg)