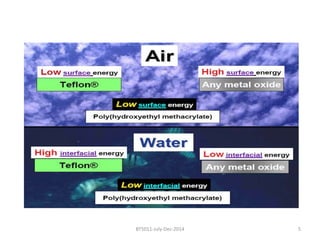
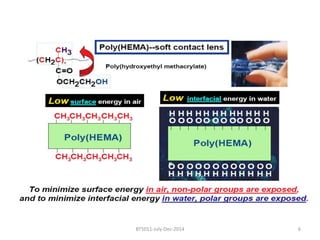
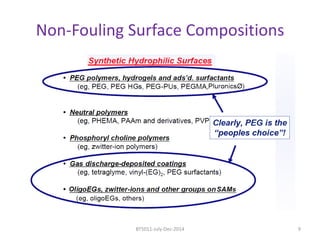
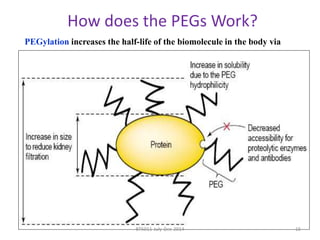
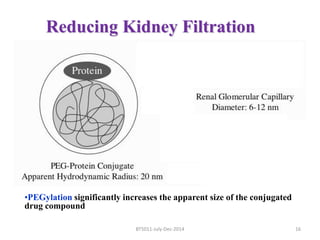
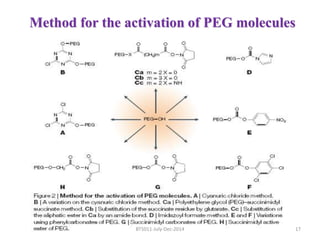
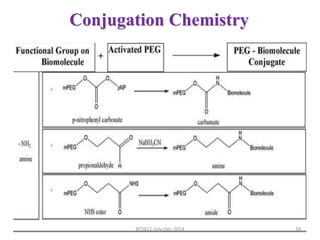
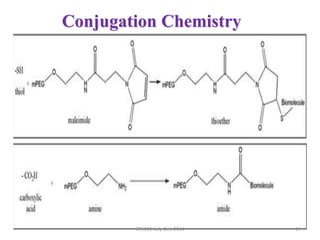
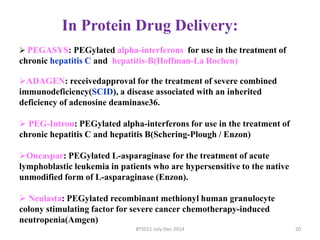
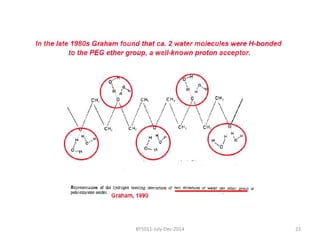
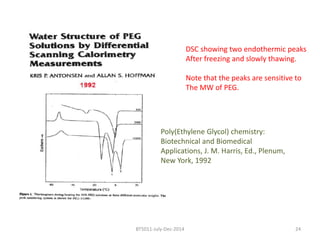
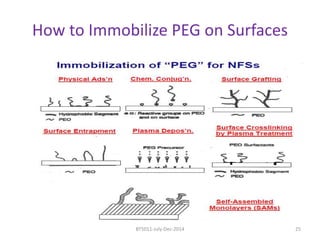
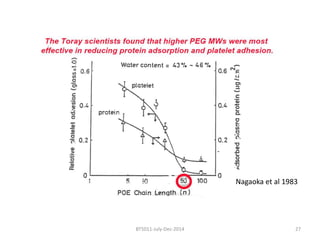
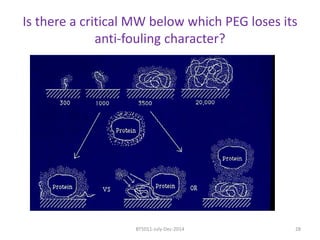
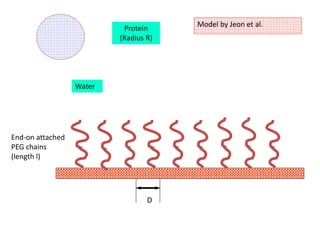
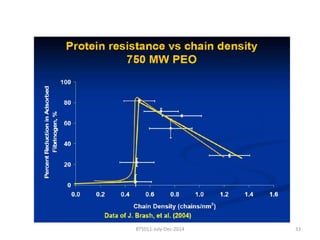
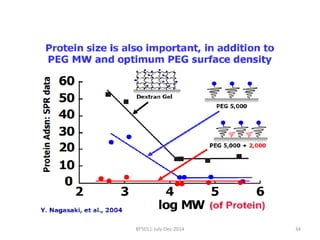
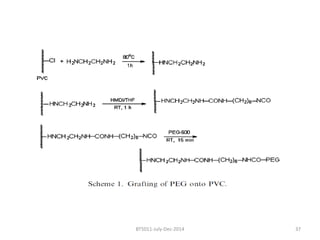
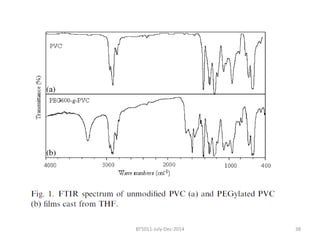
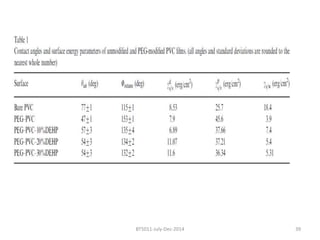
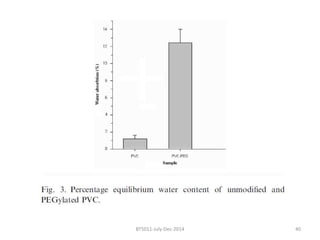
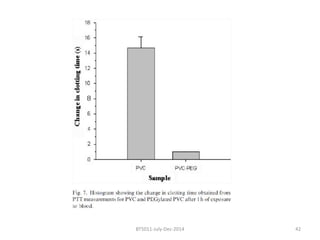
This document discusses non-fouling surfaces, specifically those using polyethylene glycol (PEG) polymers. It describes how PEGylation of drug molecules can increase their circulation time in the body by reducing kidney filtration. PEG is effective because its hydrophilic and flexible properties help form a hydration layer around coated surfaces that prevents non-specific protein adsorption. However, achieving high surface densities of PEG requires methods to overcome the excluded volume effect between tethered polymer chains. The document provides several examples of PEGylated protein drugs that have been approved for medical use.