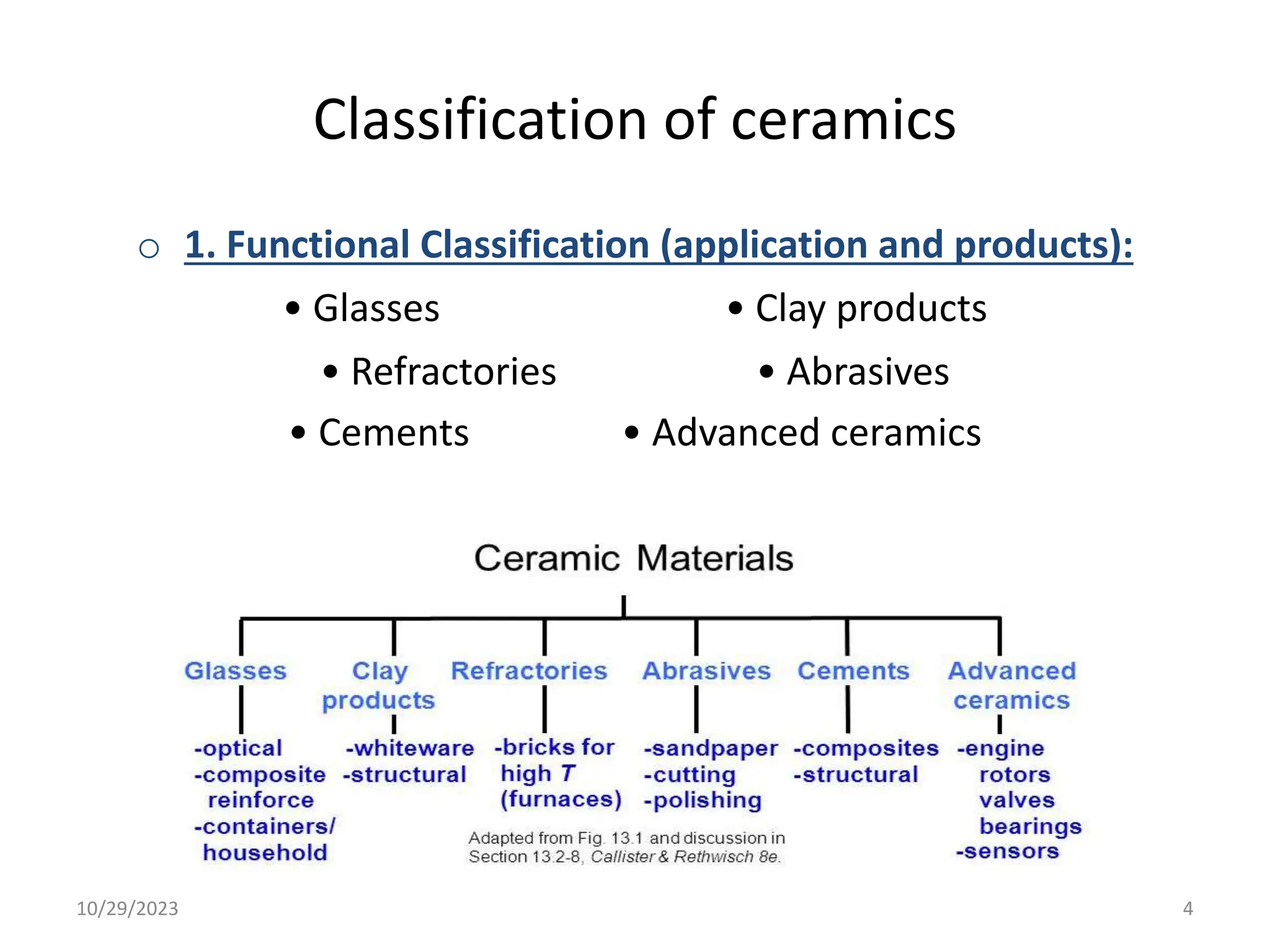
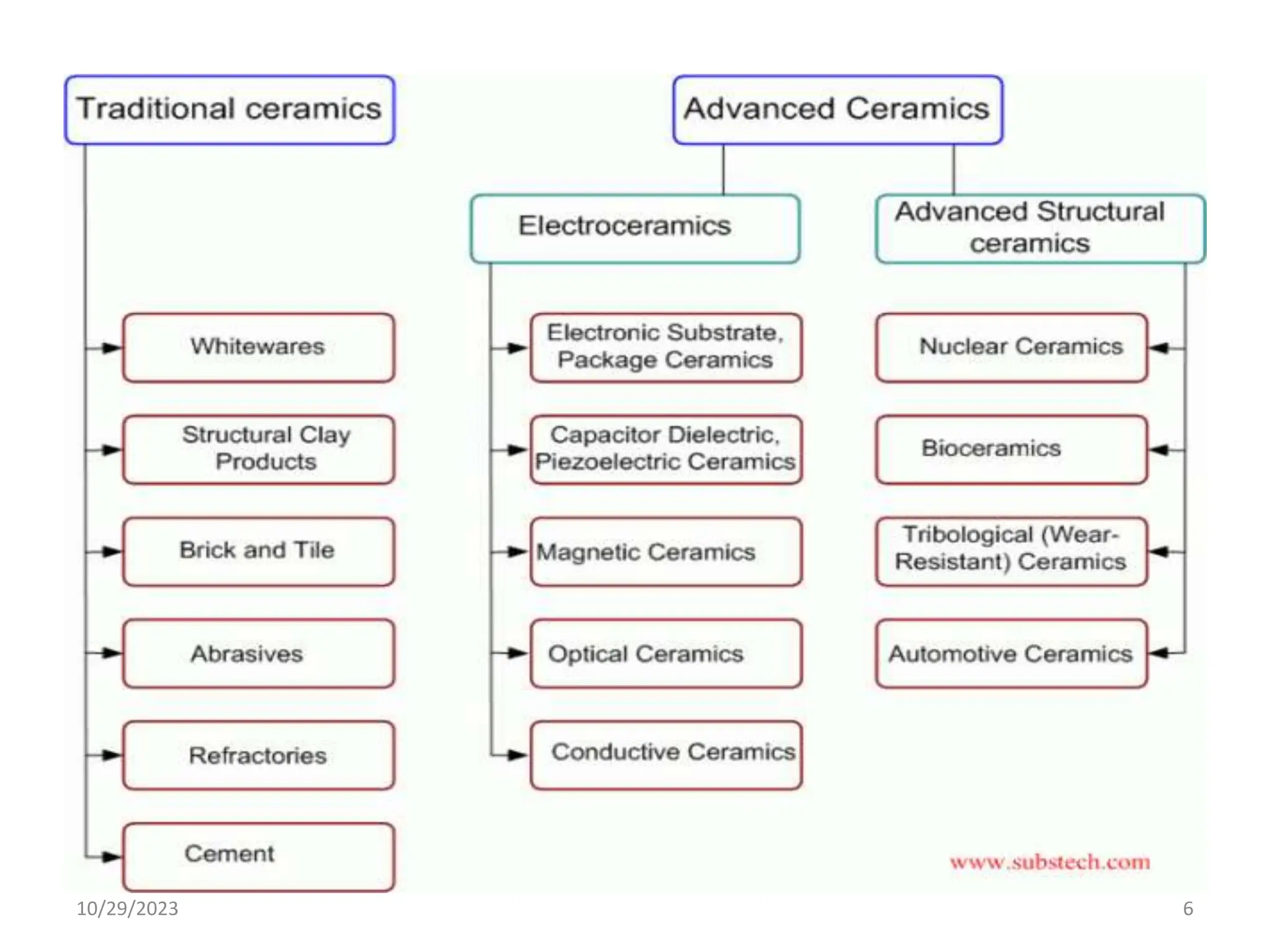
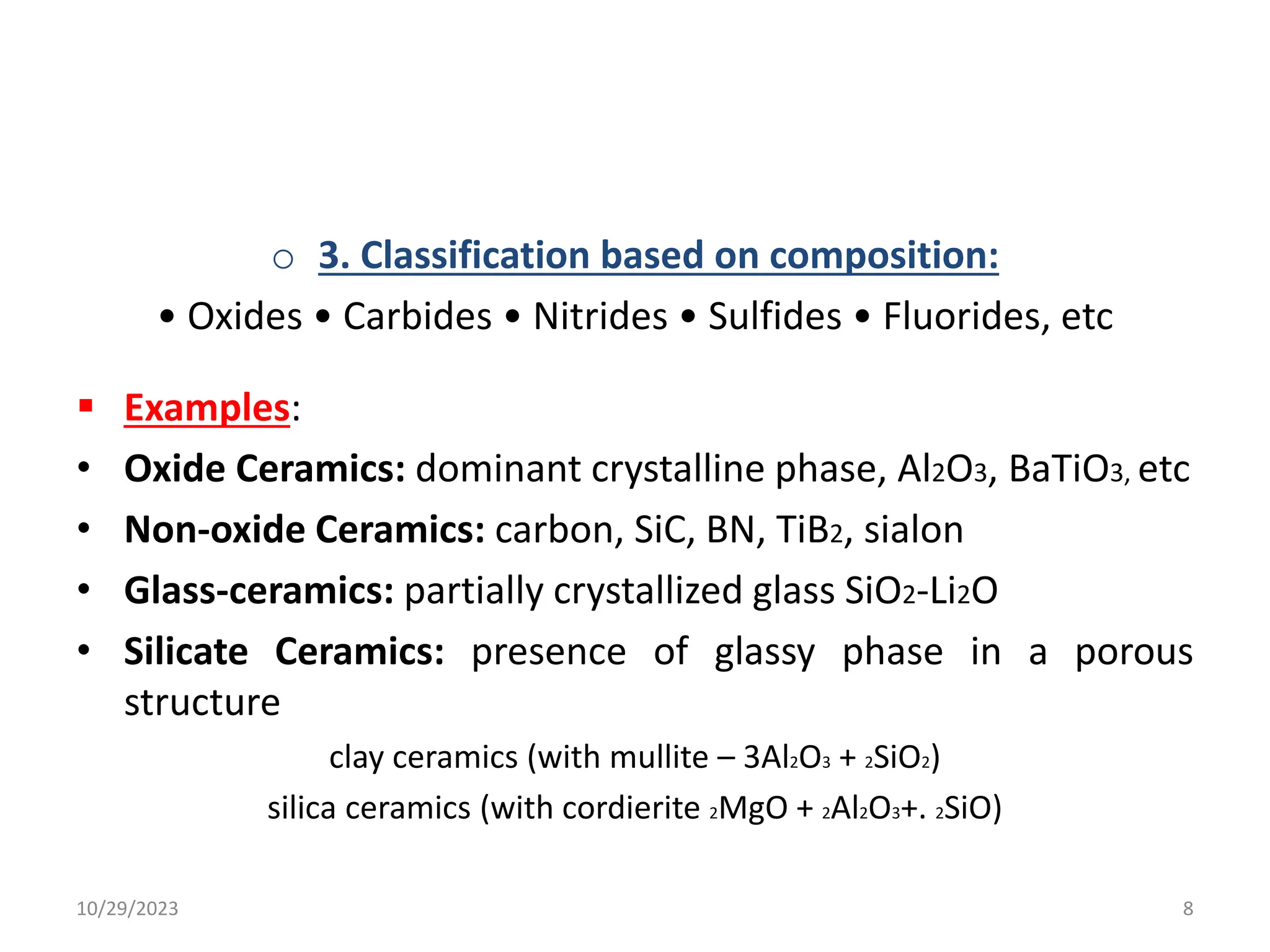
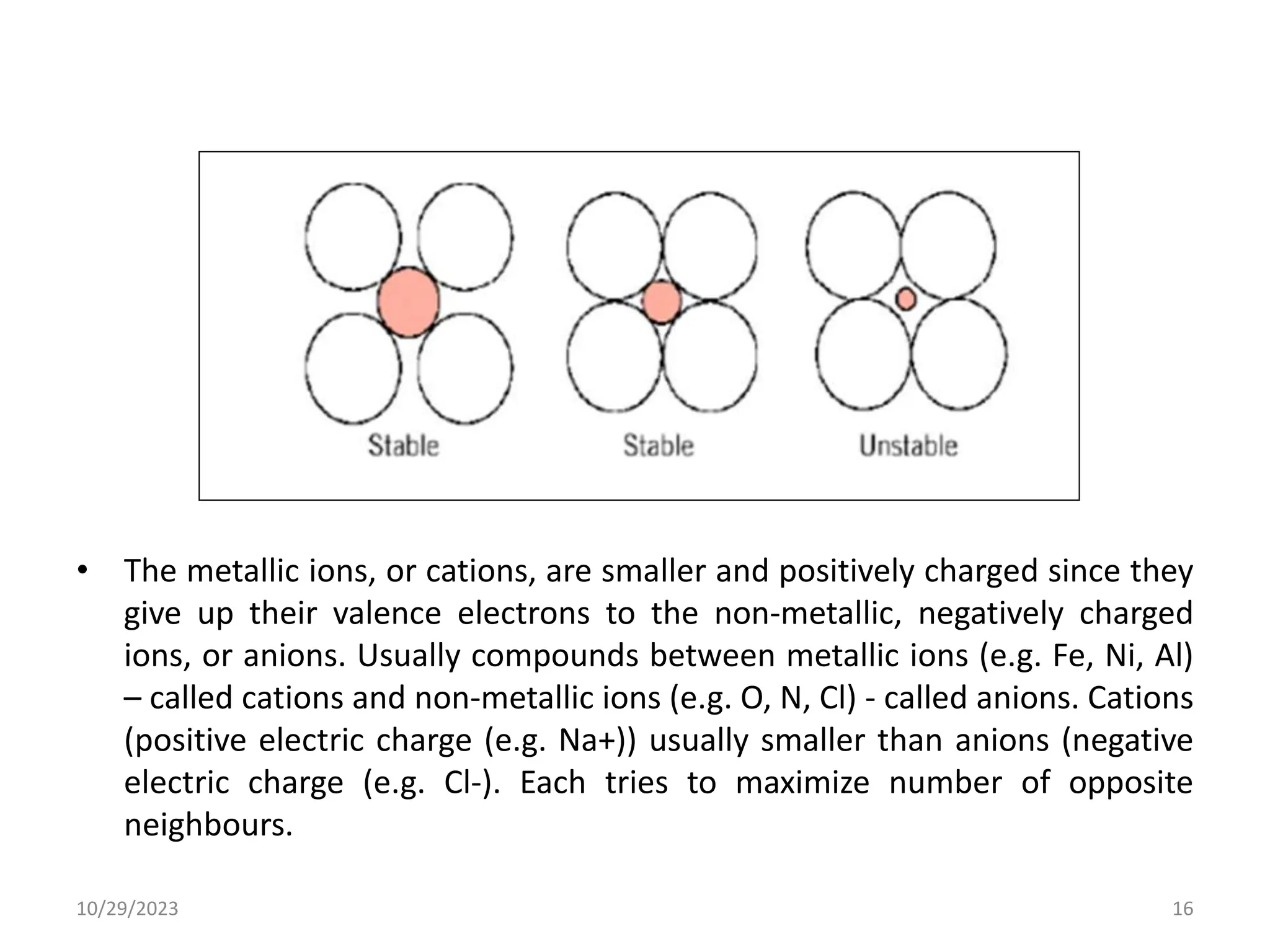
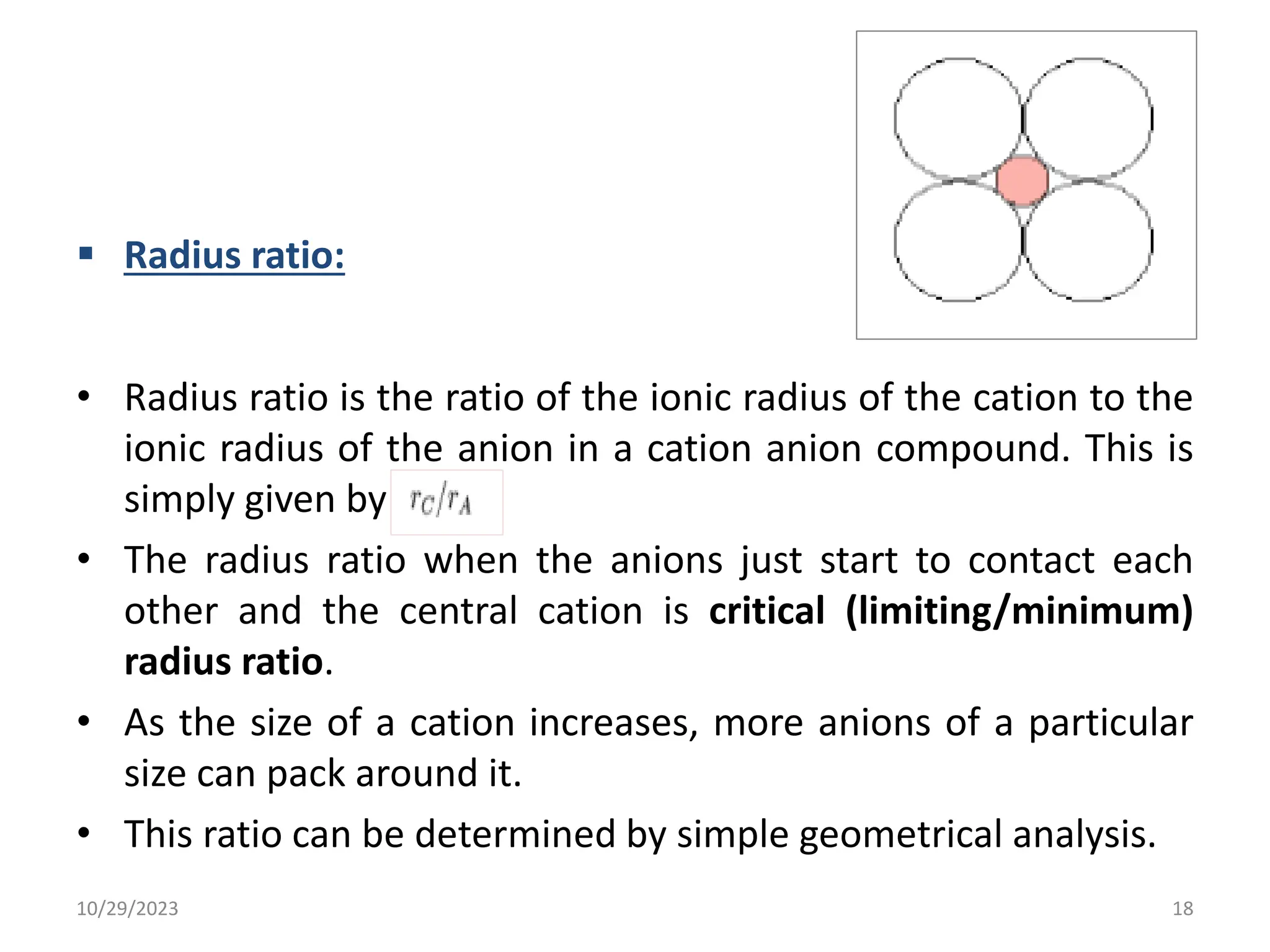
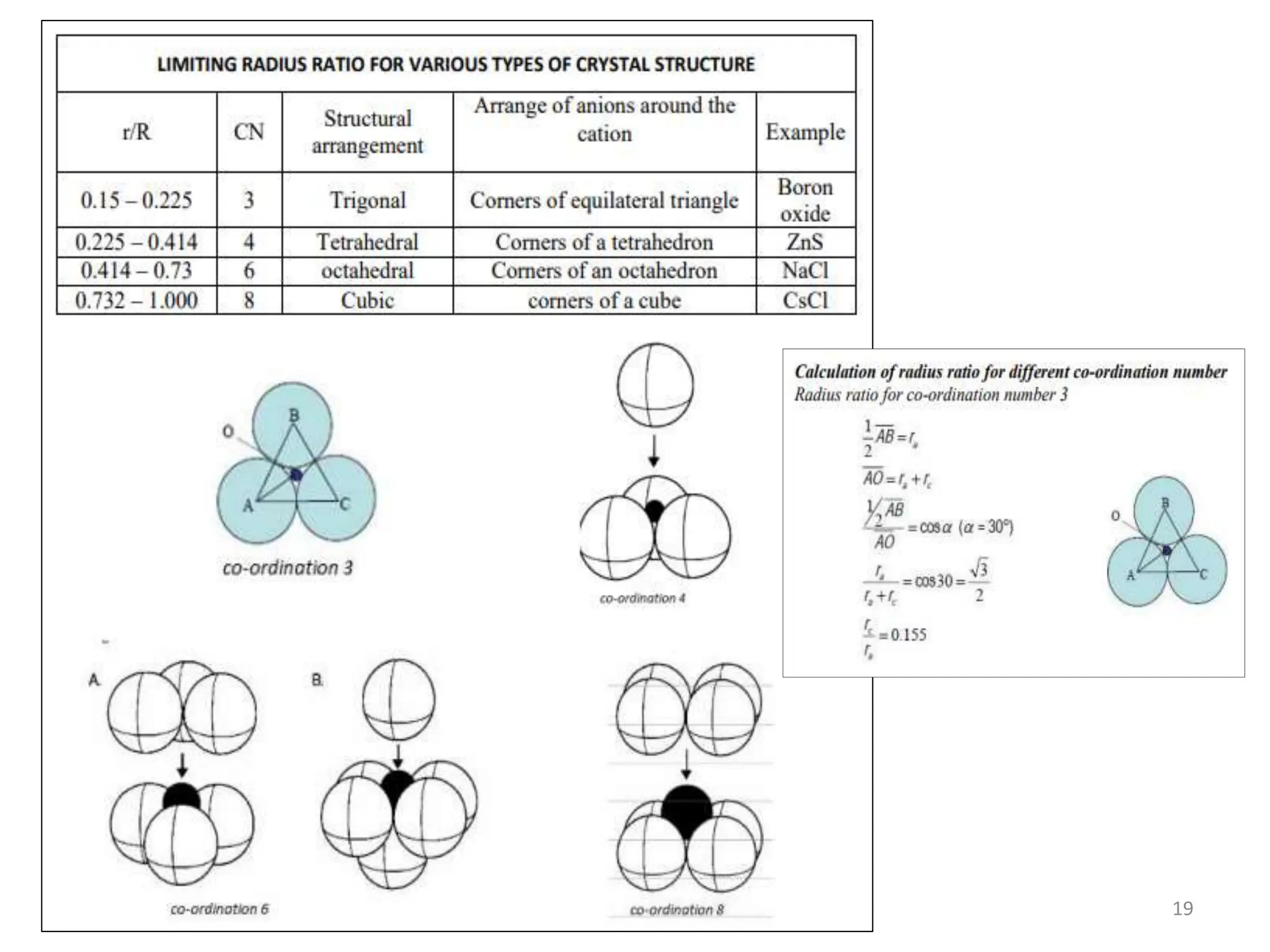
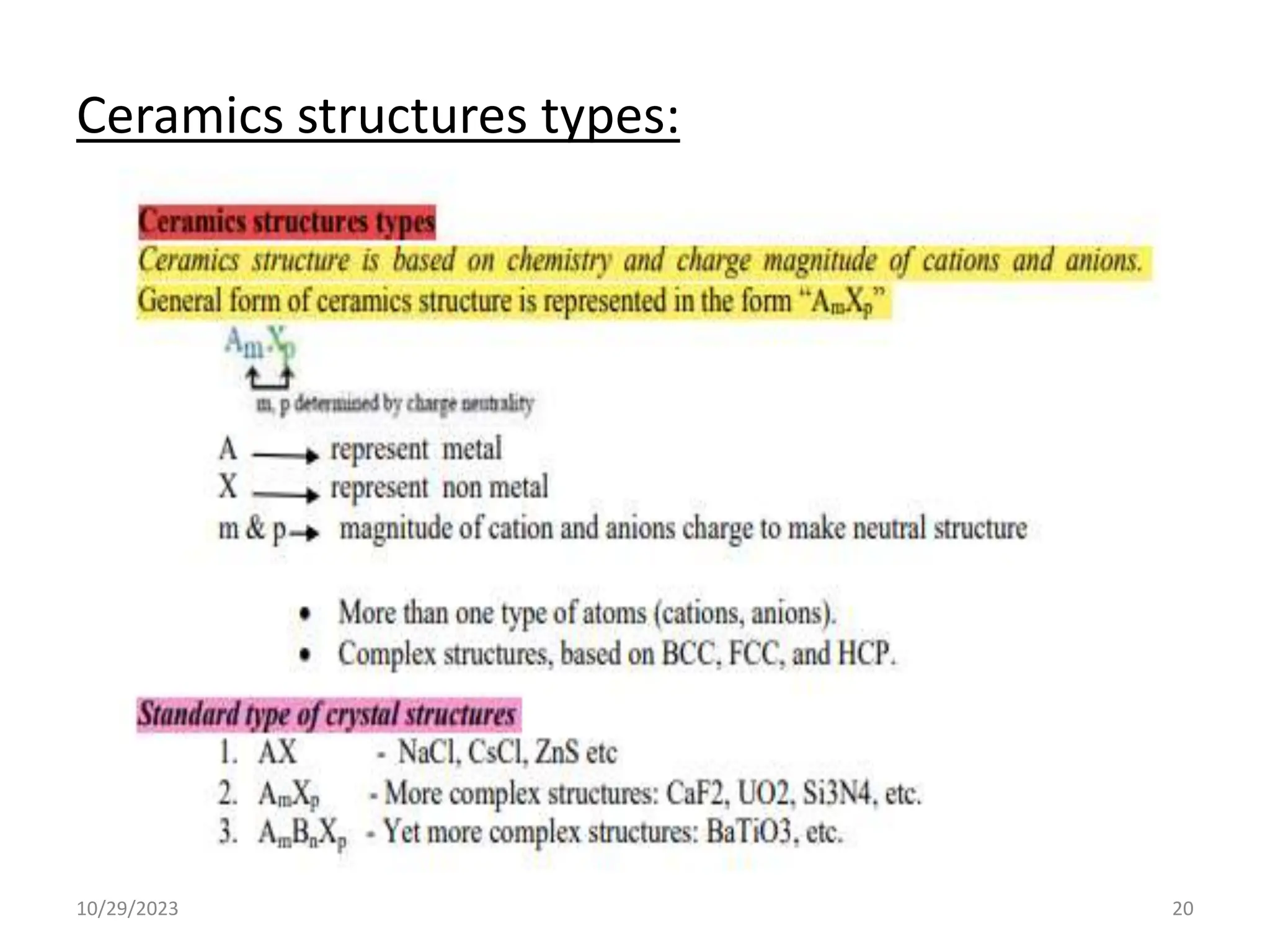
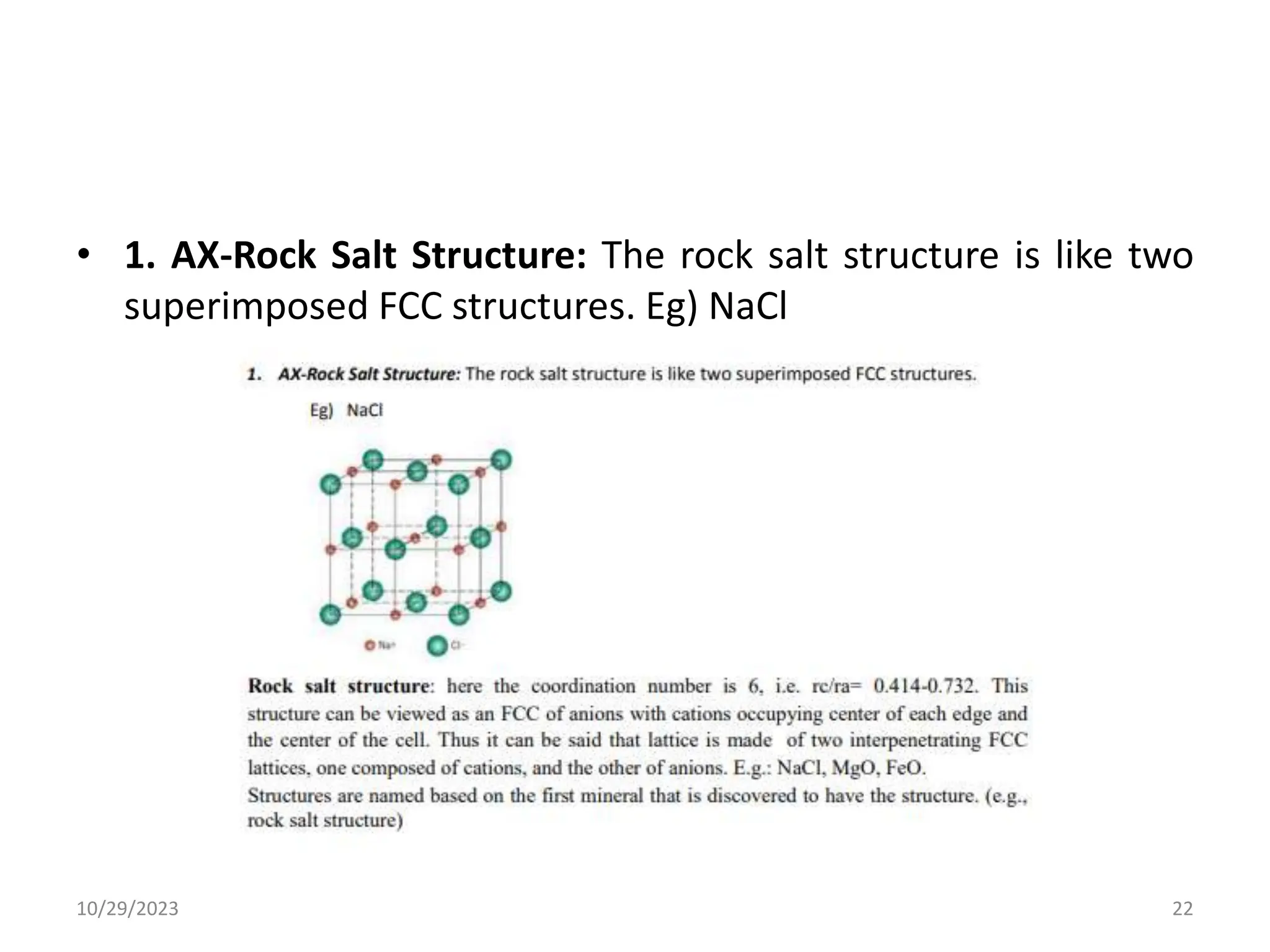
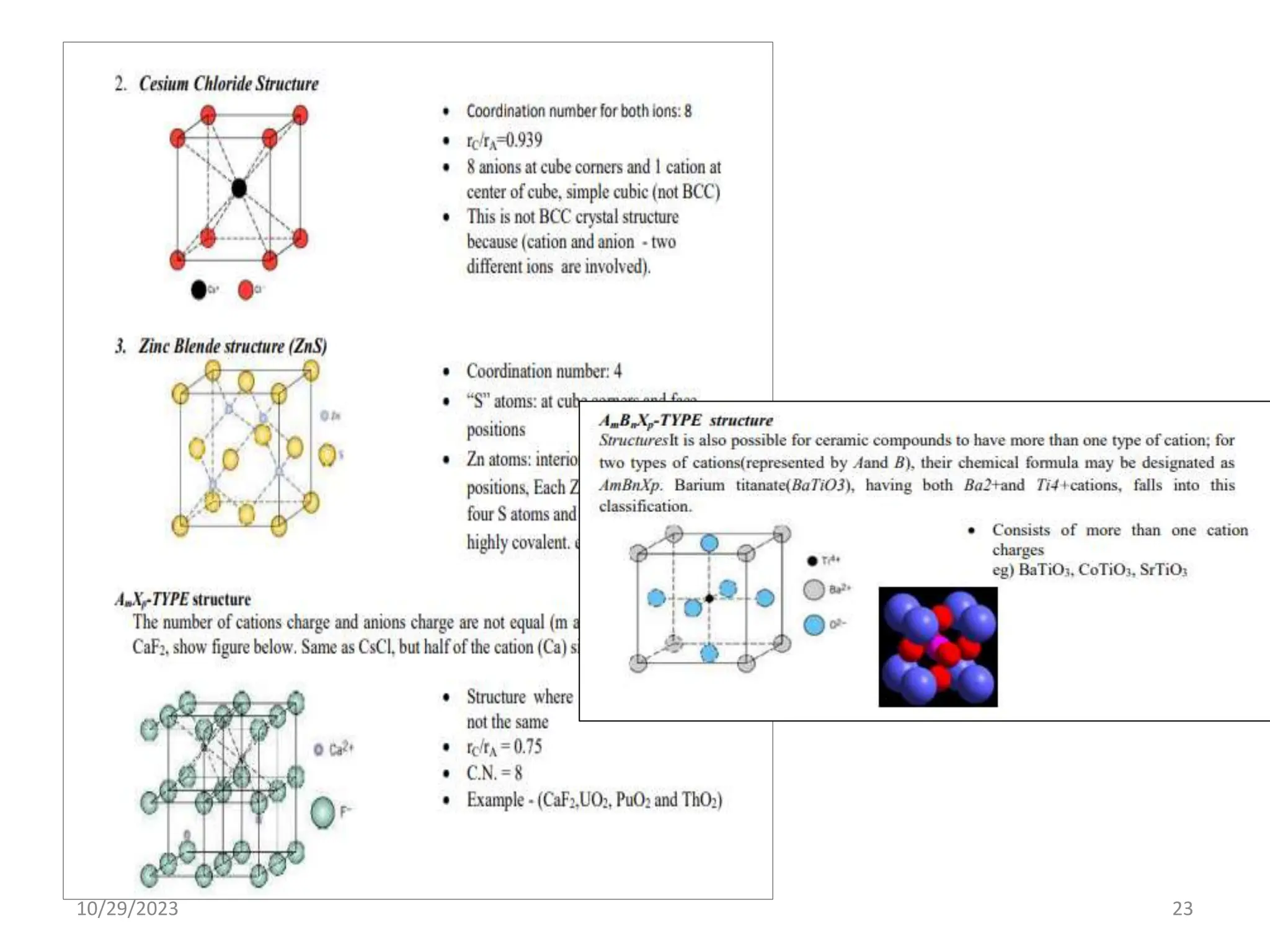
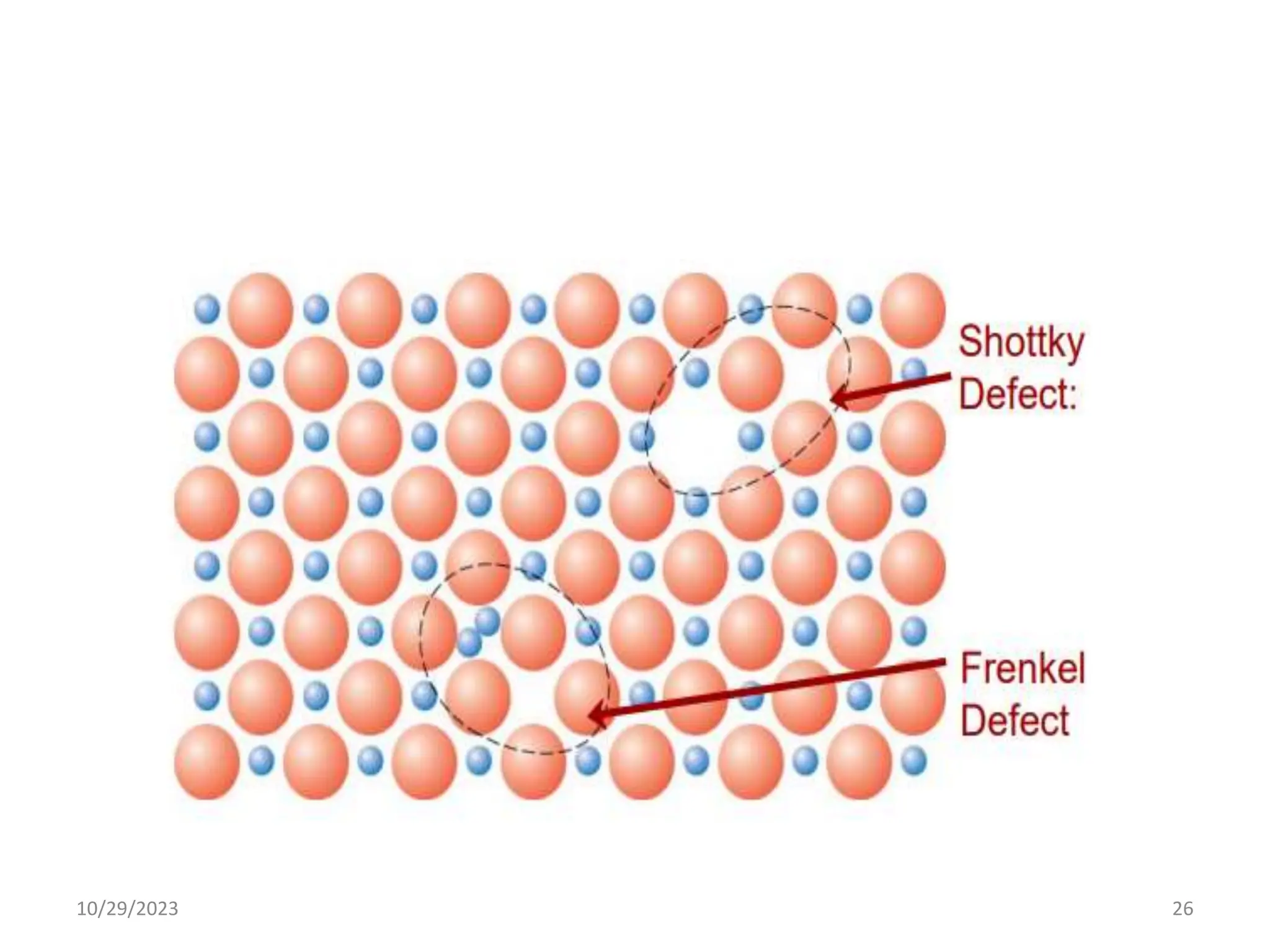
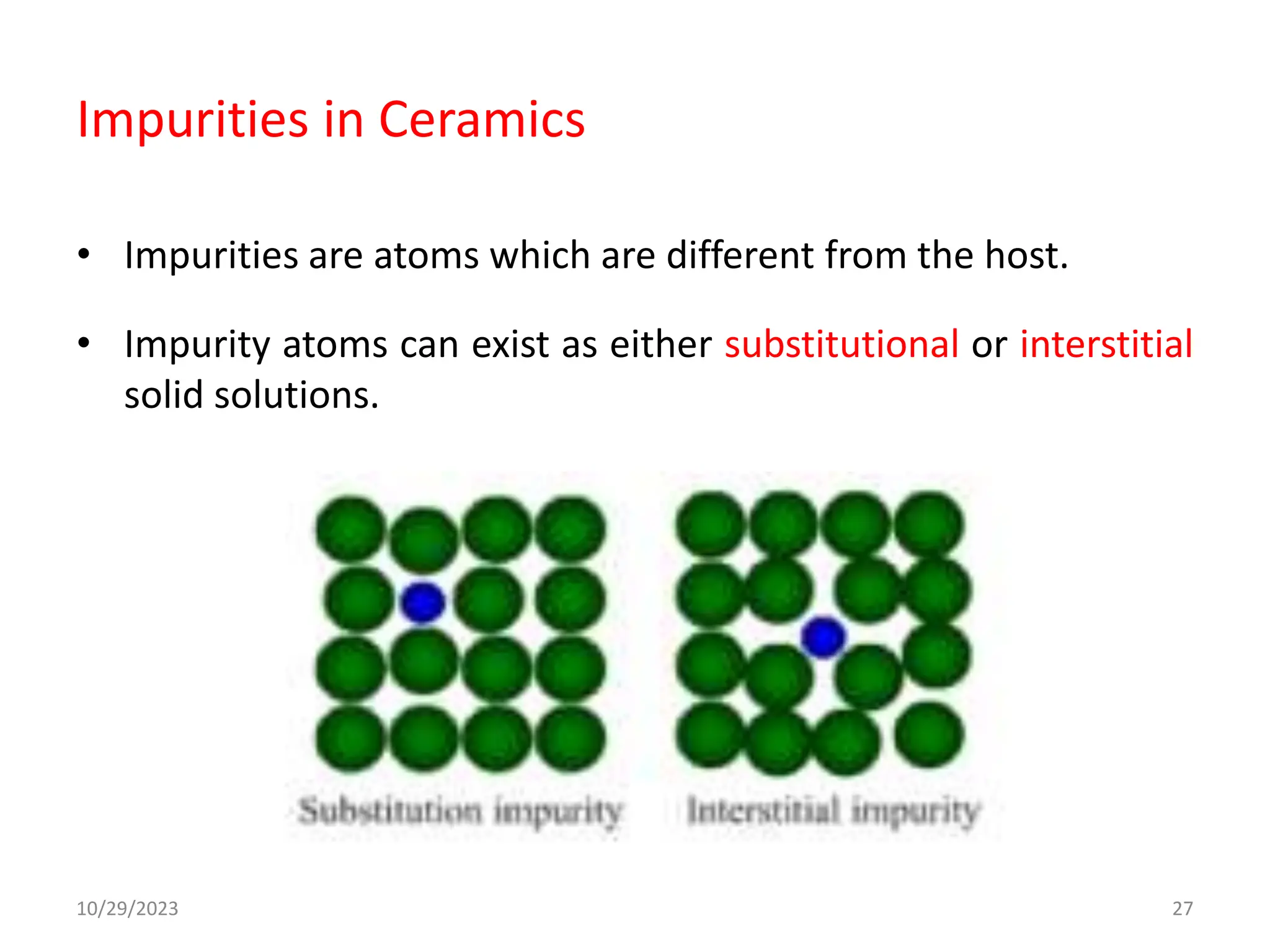
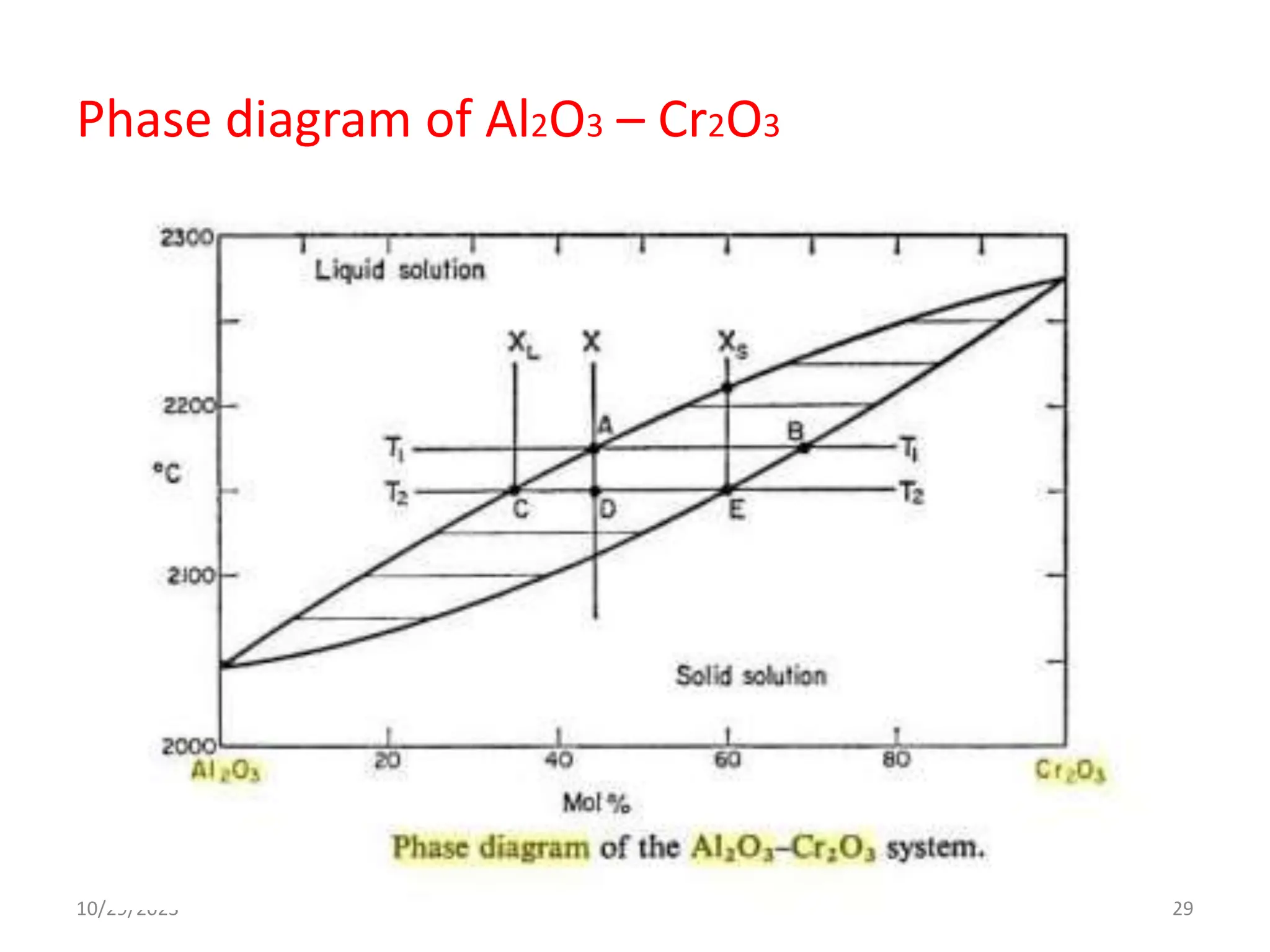
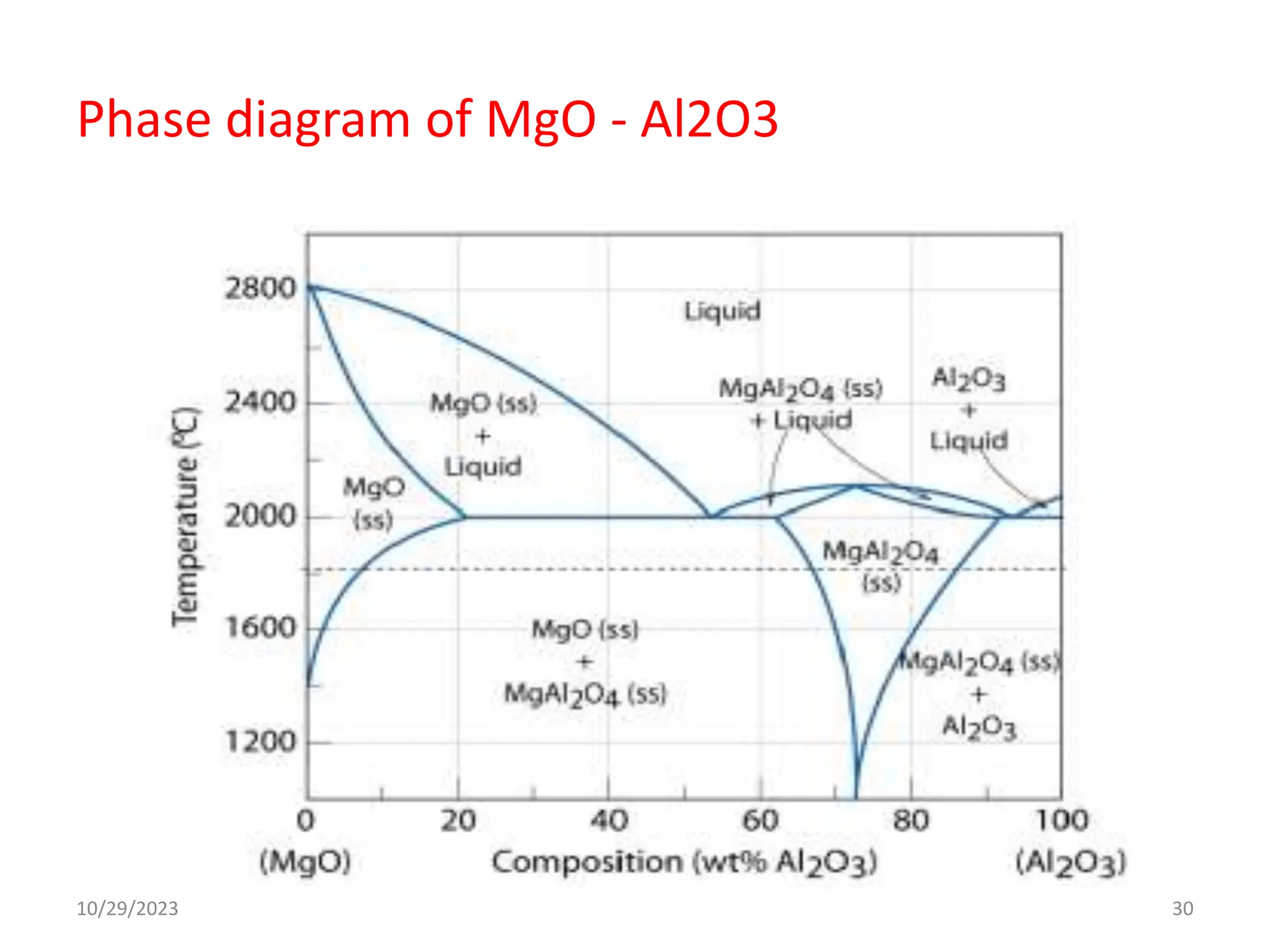
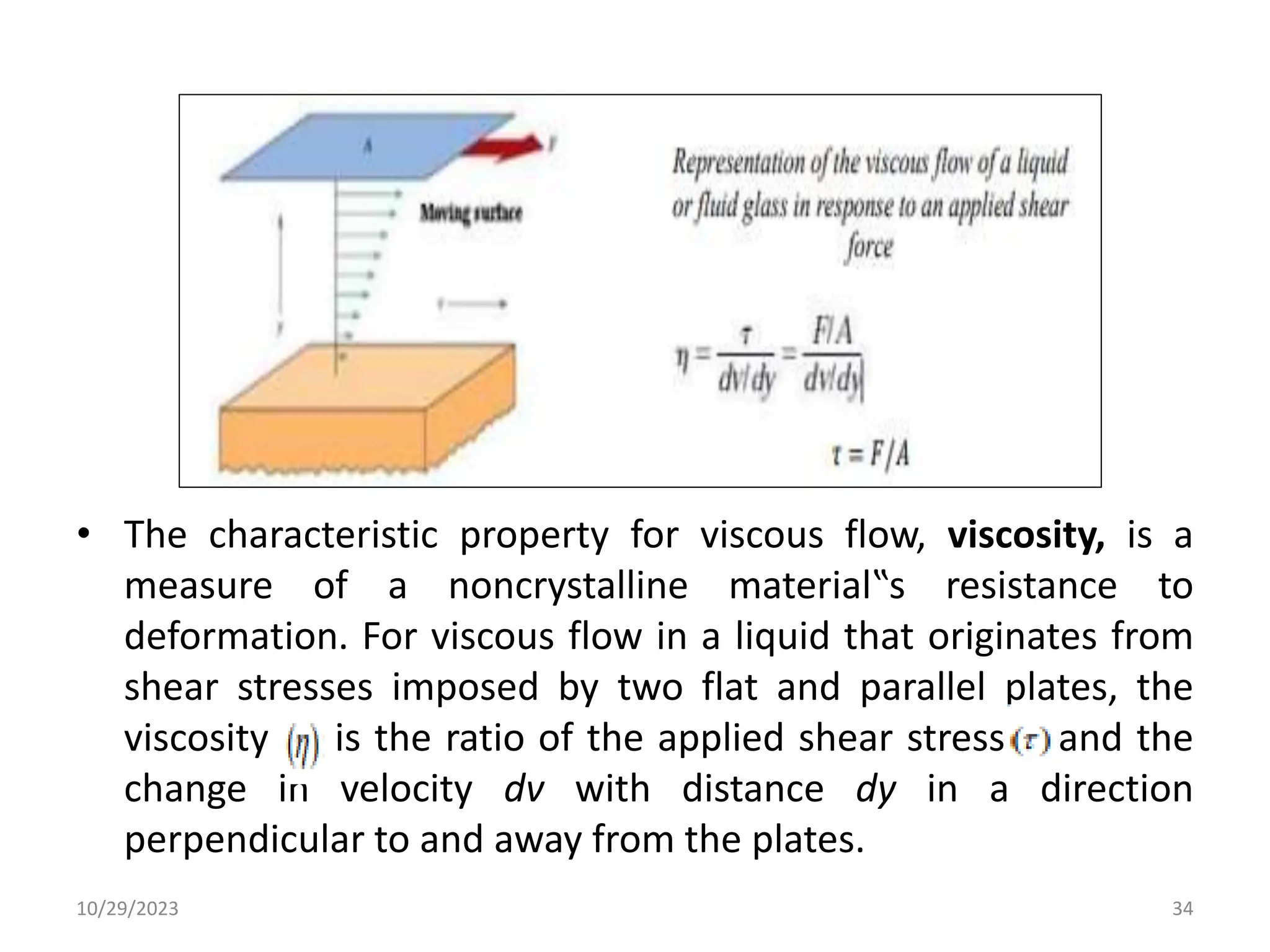
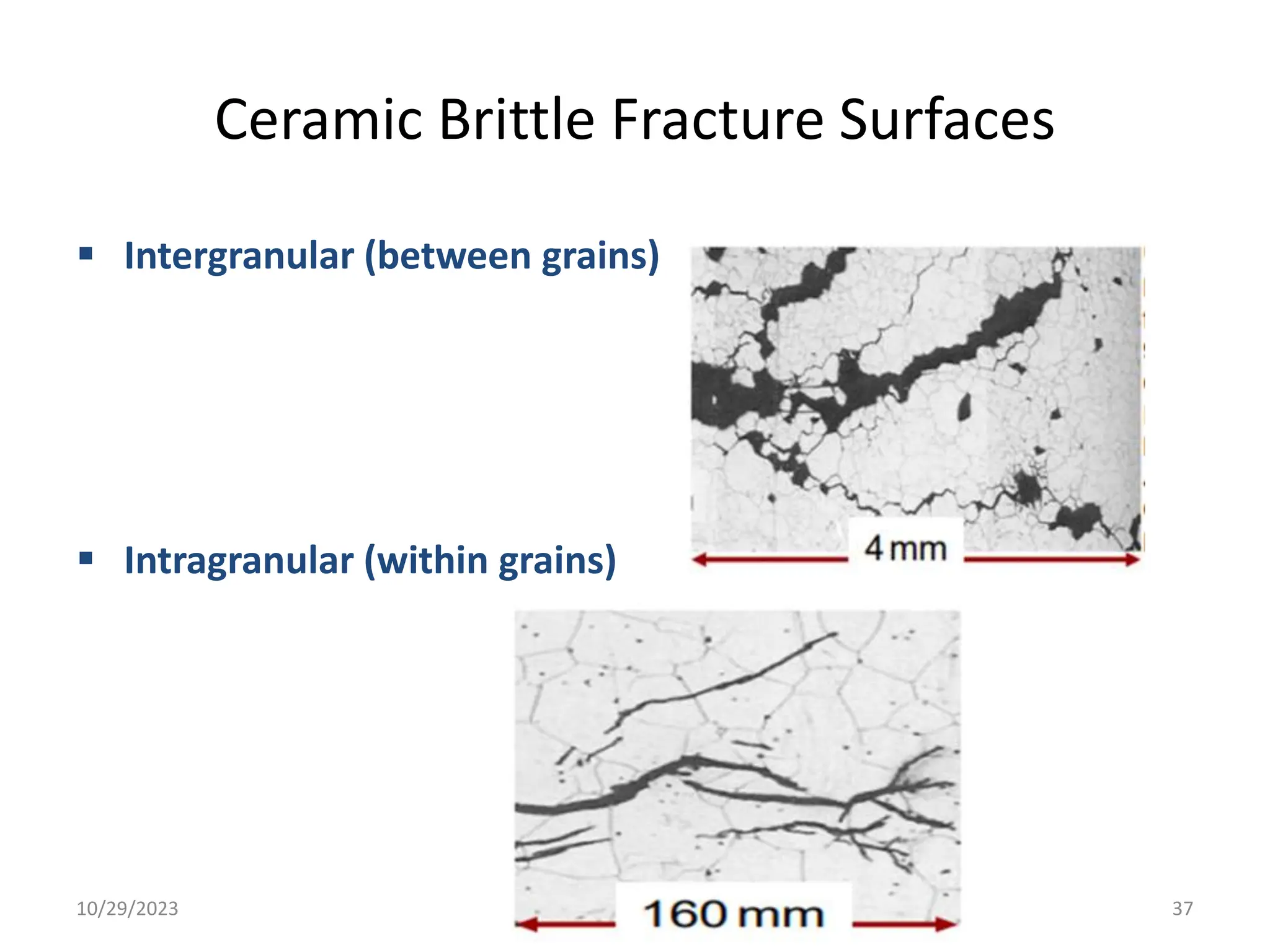
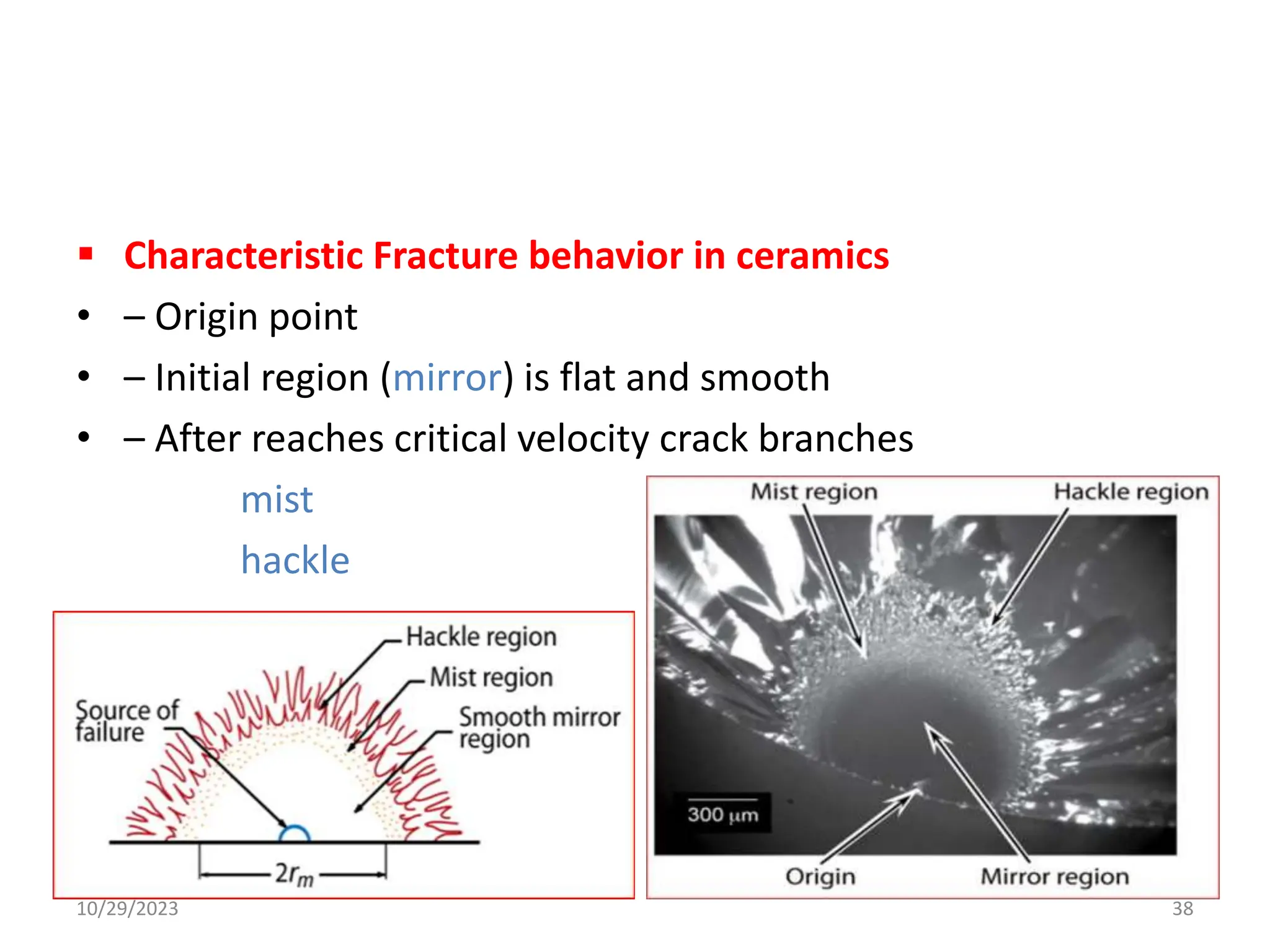
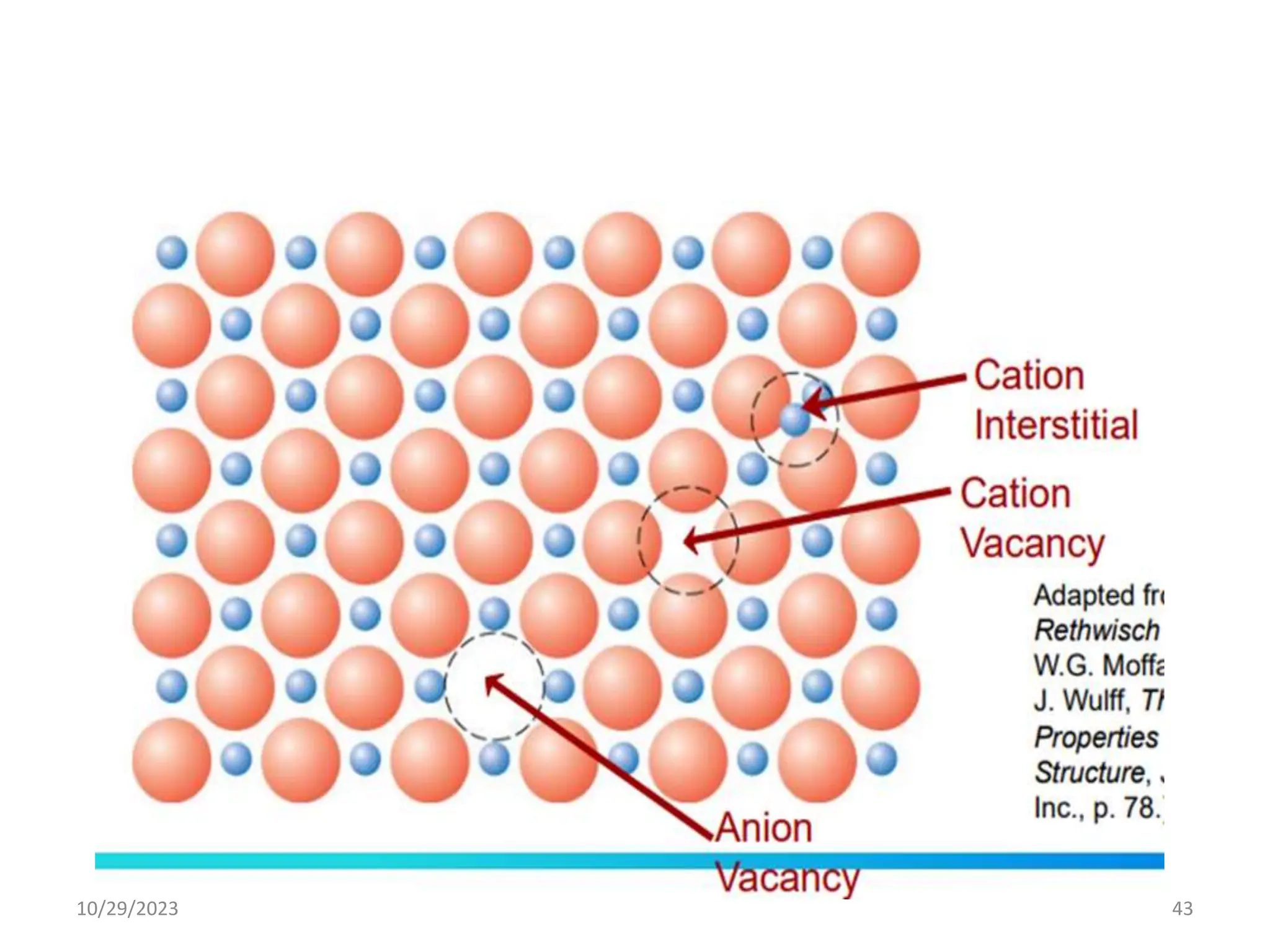
This document provides an introduction to ceramics and their classification. It discusses that ceramics are inorganic compounds composed of metallic and non-metallic elements bonded ionically or covalently. Ceramics can be classified by their application, structure, or composition. Common structures include crystalline structures like rock salt and zinc blend. Ceramic properties include hardness, high melting temperatures, and electrical/thermal insulation. Due to these properties, ceramics find applications in areas like aerospace, automotive, electronics and more. The document also discusses ceramic crystal structures, imperfections, and phase diagrams.