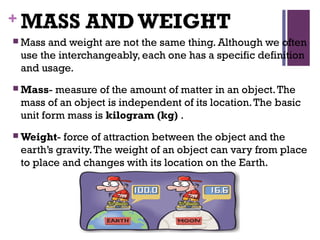
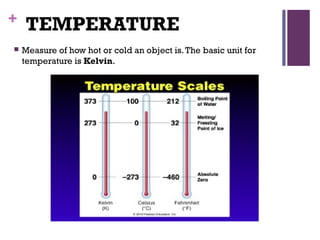
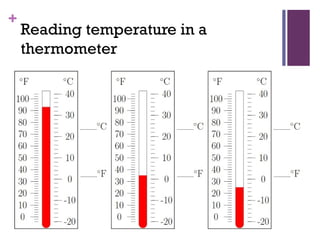
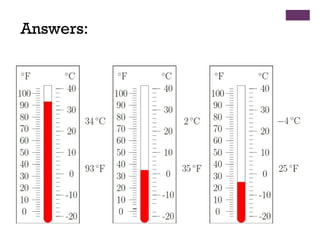
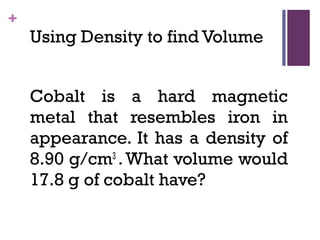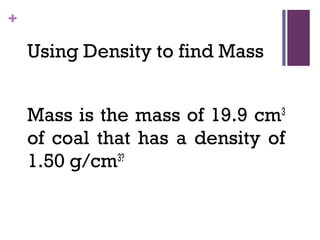This document provides an introduction to physical science. It begins by defining science and listing the main branches - biological science, physical science, and social science. Biological science deals with living things, social science deals with human behavior and societies. Physical science deals with non-living things, their composition, nature, characteristics, and changes.
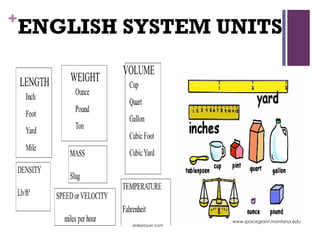
The main branches of physical science are then defined: chemistry studies matter and its properties/structure/changes, physics studies matter and energy/their interactions, astronomy studies the universe/heavenly bodies, geology studies Earth materials/structures/processes, and meteorology studies the atmosphere/weather/climate.
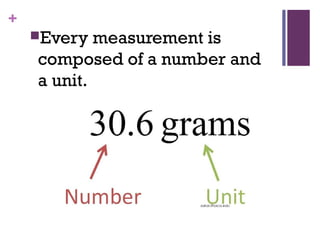
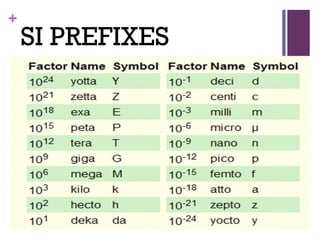
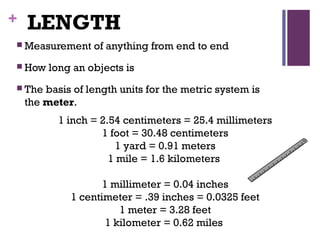
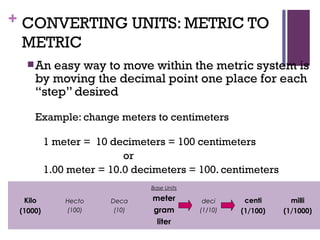
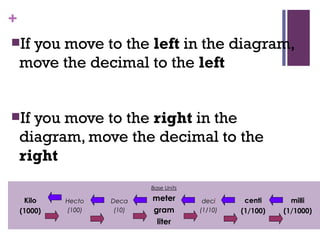
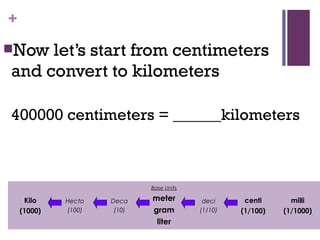
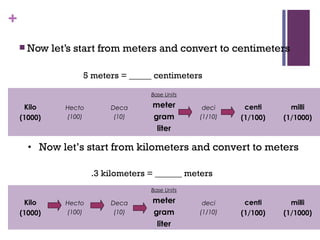
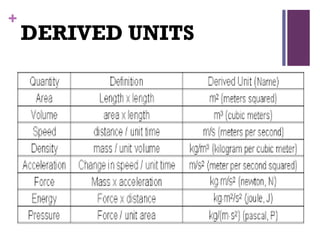
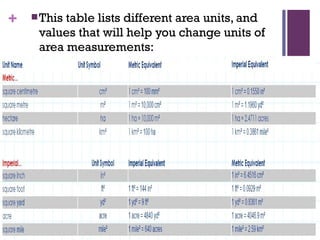
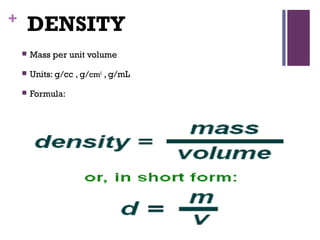
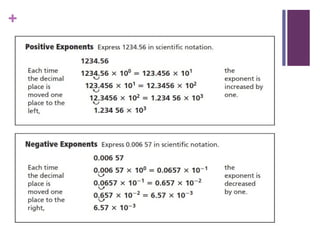
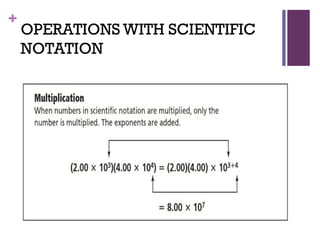
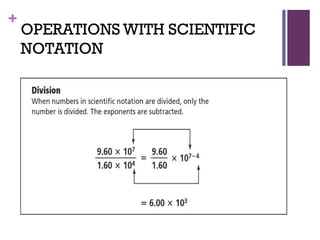
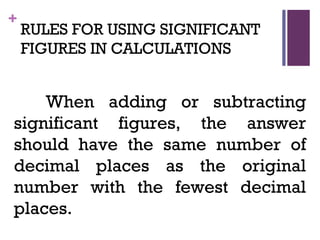
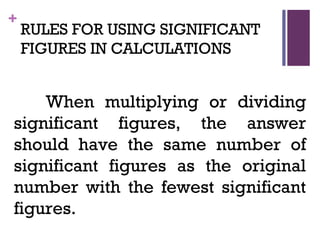
The document then moves to a chapter about measurement, defining it as collecting quantitative data by