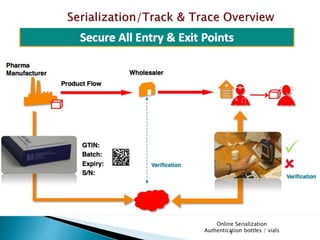
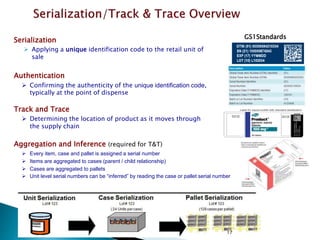
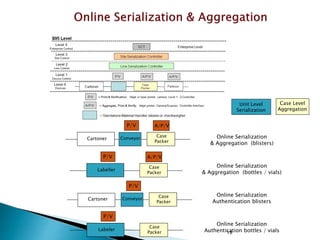
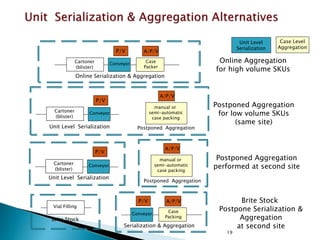
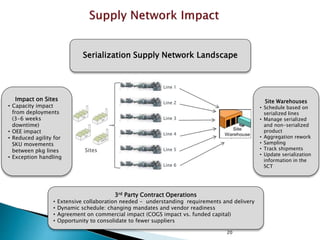
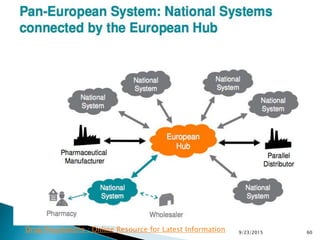
This presentation summarizes the key requirements of the EU Delegated Regulation on safety features for medicinal products. It describes the obligations to prevent falsified medicines, including applying a unique identifier and anti-tampering device. It outlines the core elements of the regulation, such as the composition of the unique identifier, end-to-end verification system, and establishment of repositories to store unique identifiers. The regulation aims to implement traceability systems to securely track medicinal products and help prevent entry of falsified medicines into the legal supply chain.