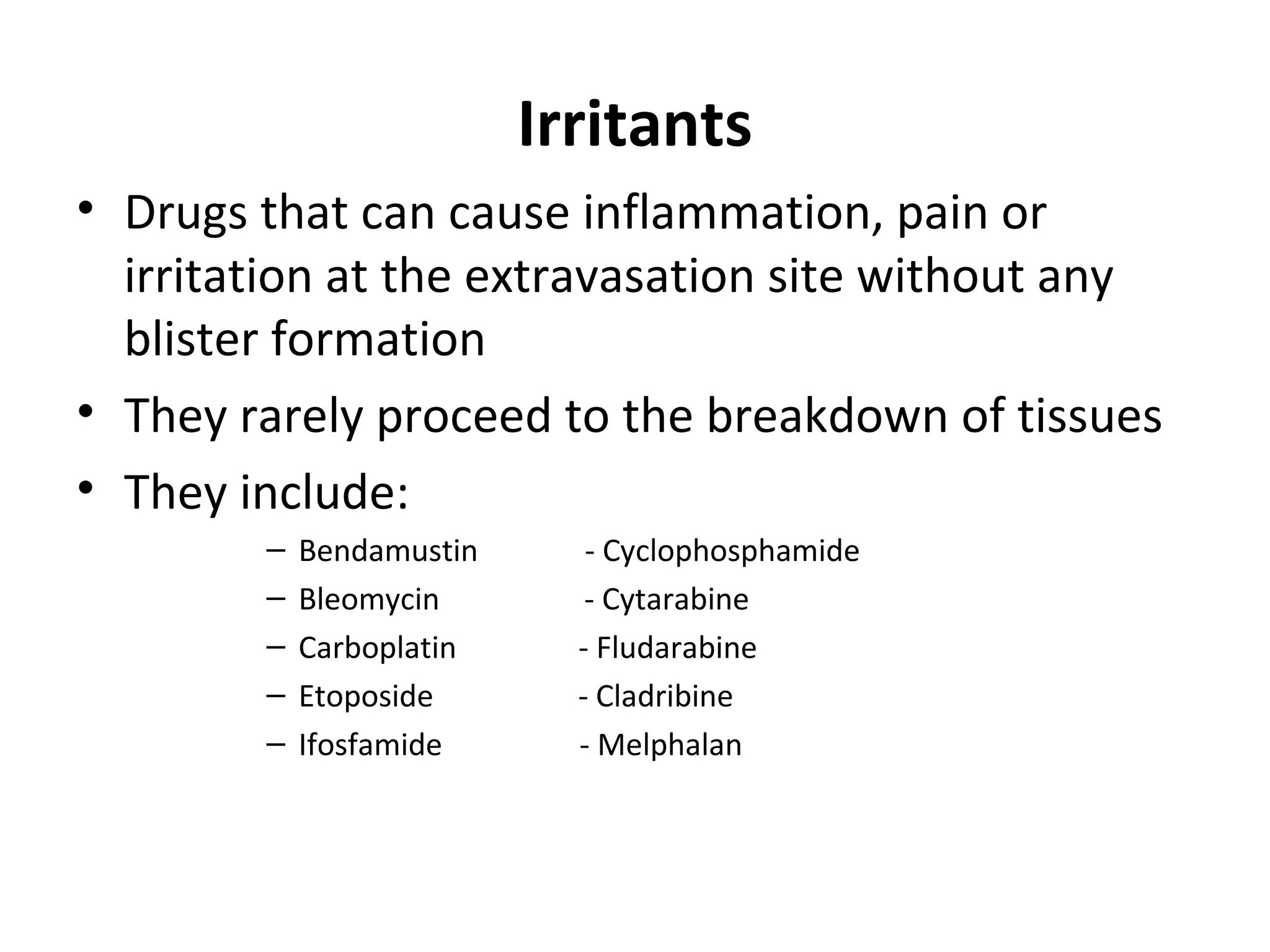
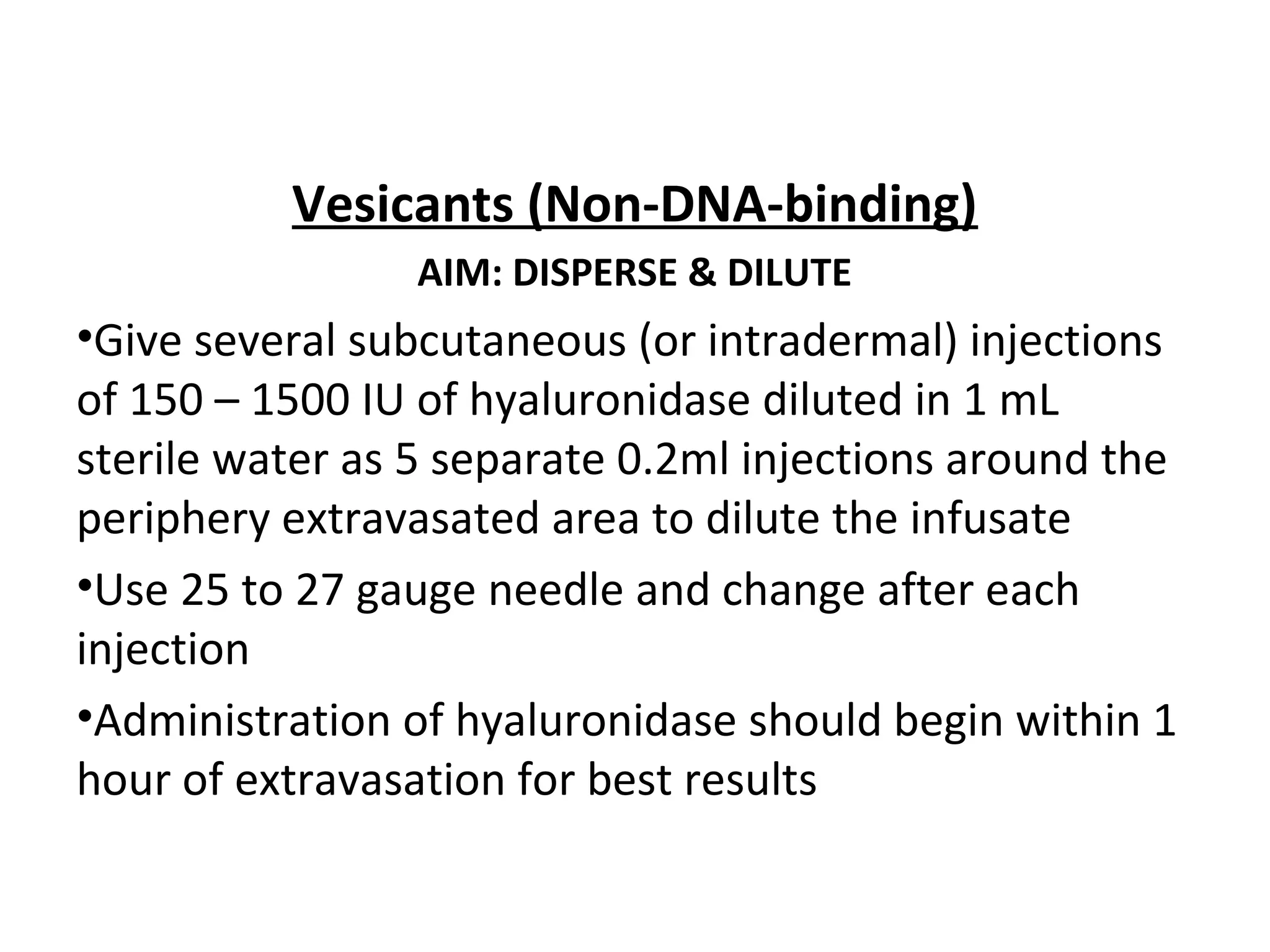
Extravasation is the accidental leakage of intravenous drugs into surrounding tissues from a vein. This can cause immediate pain and inflammation and potentially tissue damage or necrosis depending on the drug. The risk of extravasation is between 0.1-6% for peripheral lines and 0.26-4.7% for central lines. Drugs are classified based on their damage potential from vesicants, which can cause blistering, to exfoliants, irritants, inflammitants and neutrals. Management involves stopping the infusion, aspirating drug if possible, and then treatments ranging from dispersing and diluting for non-DNA binding vesicants to localizing and neutralizing for DNA binding vesicants. Prevention