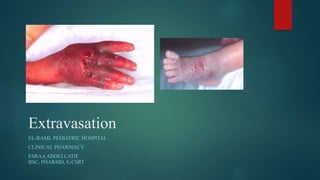
1) Extravasation occurs when a drug escapes into the extravascular space through vessel leakage or direct infiltration, potentially causing tissue damage or necrosis.
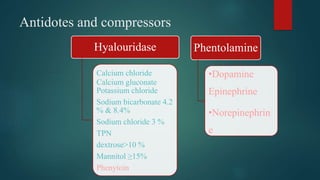
2) Symptoms of extravasation appear within 30 minutes to 12 hours and range from skin irritation to tissue damage, depending on the type of drug (irritant or vesicant).
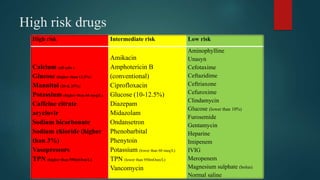
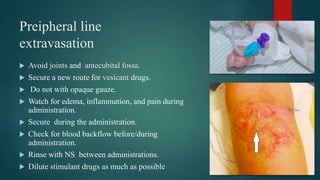
3) Risk factors for extravasation include drug properties, patient factors like vein availability, and environmental factors. Proper prevention, recognition, and management are needed to minimize risks of extravasation.