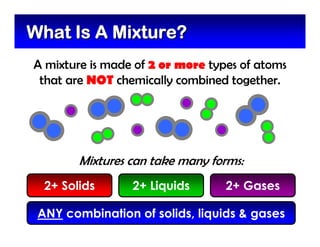
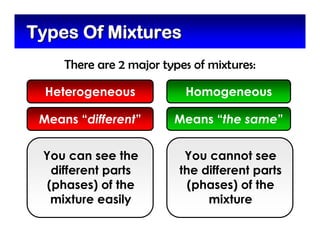
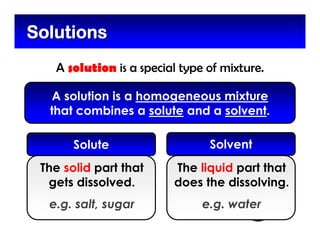
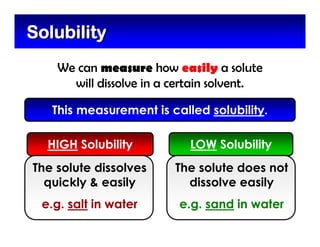
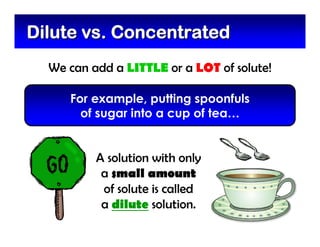
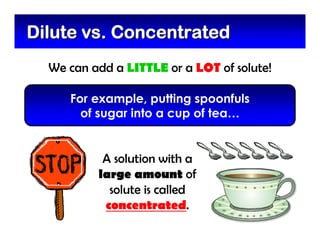
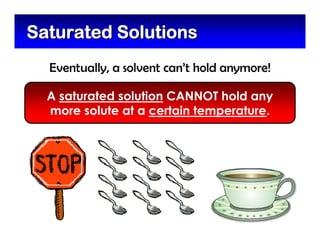
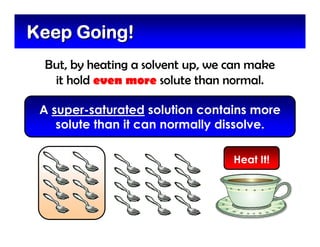
This document defines and describes mixtures and solutions. It states that a mixture is made of two or more substances that are not chemically combined and can exist as solids, liquids, gases, or combinations. Mixtures are either heterogeneous, where the phases can be seen, or homogeneous, where they cannot. A solution is a special type of homogeneous mixture containing a solute dissolved in a solvent. It discusses how to make solutions by dissolving a solute in a solvent, and defines concepts like solubility, dilute vs concentrated solutions, saturated solutions, and super-saturated solutions.