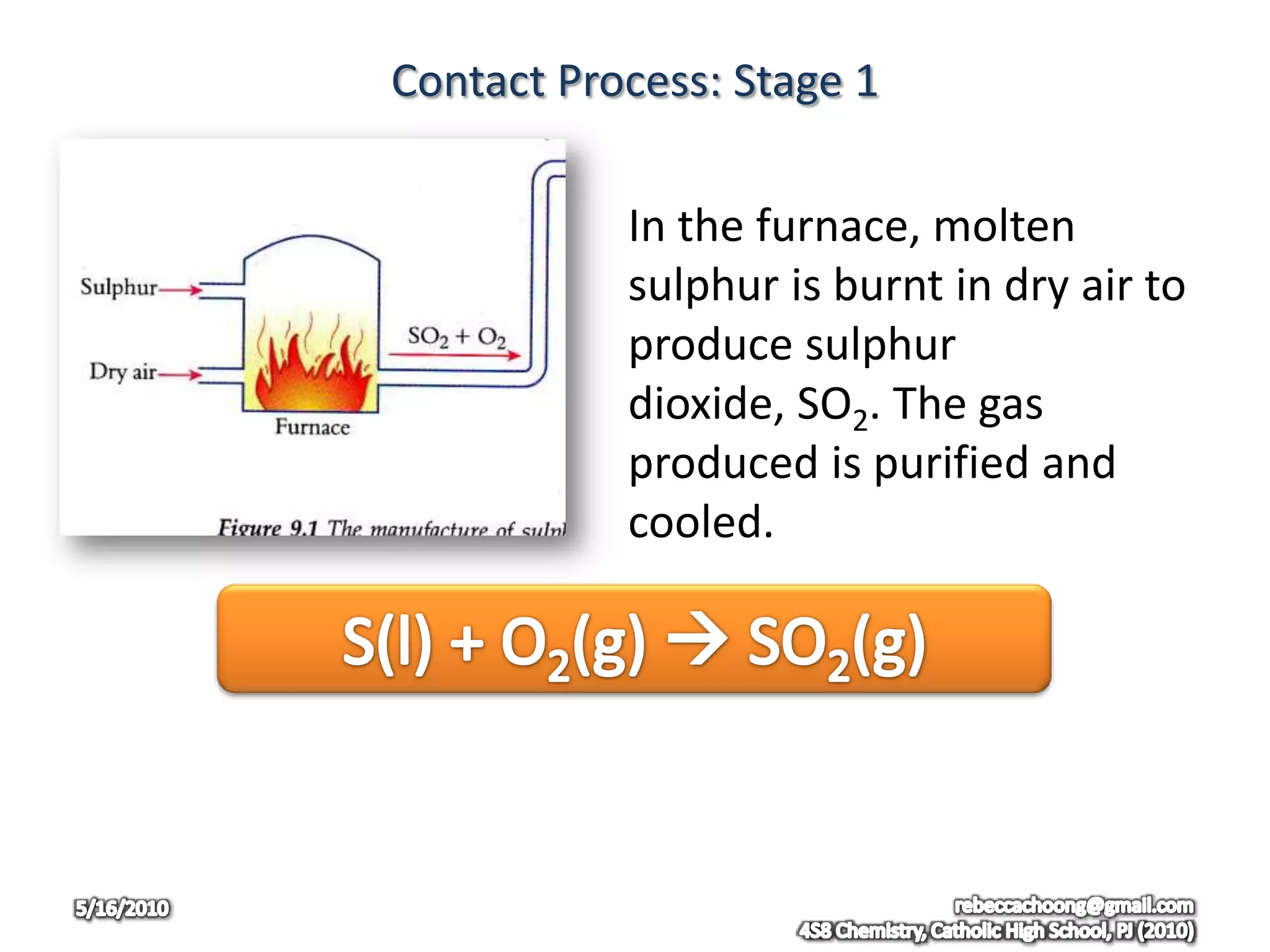
The document discusses the uses of sulphuric acid and the contact process for manufacturing sulphuric acid. Specifically, it describes the three main stages of the contact process: (1) burning sulphur to produce sulphur dioxide, (2) passing sulphur dioxide and oxygen over a vanadium catalyst to produce sulphur trioxide, and (3) reacting sulphur trioxide with concentrated sulphuric acid and water to produce more sulphuric acid. It also discusses some common uses of sulphuric acid and issues related to sulphur dioxide emissions.














![FAQ 1The two reaction in the third stage are equivalent to adding sulphur trioxide, SO3, directly to waterSO3 (g)+ H2O(l) H2SO4(l)Then why can’t we just skipped concentrated sulphuric acid step [thus not forming oleum]?17/5/2010rebeccachoong@gmail.com4S8 Chemistry, Catholic High School, PJ (2010)](https://image.slidesharecdn.com/class2-contactprocesshaberprocessandalloy-100516201448-phpapp01/75/Chapter-9-Contact-Process-Haber-Process-and-Alloy-16-2048.jpg)























