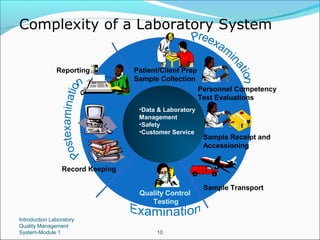
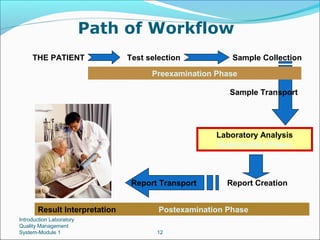
1. A quality management system for a medical laboratory seeks to efficiently achieve the objectives of providing accurate test results to physicians and contributing to patient care.
2. Key aspects of a quality management system include personnel management, equipment management, process control, purchasing and inventory, and continuous improvement.
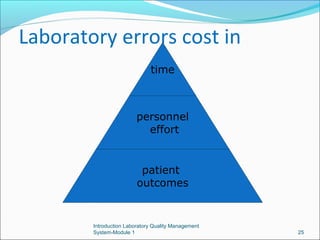
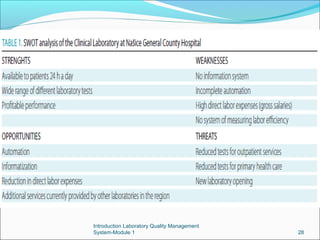
3. Implementing a quality management system can help detect and prevent errors, saving time, personnel costs, and improving patient outcomes compared to an error-prone laboratory.