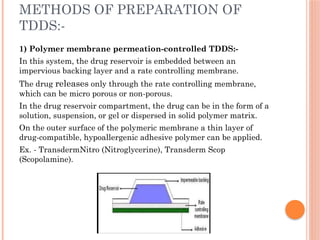
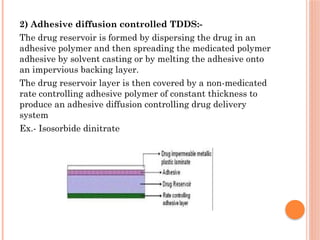
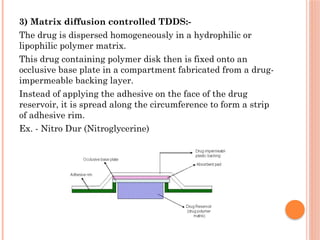
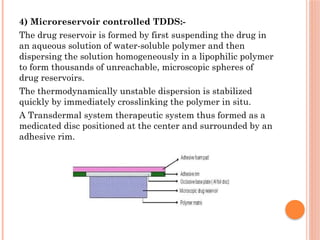
The document provides a comprehensive overview of transdermal drug delivery systems (TDDS), detailing their formulation components, including drug reservoirs, polymers, penetration enhancers, adhesives, and backing layers. It outlines methods of preparation and evaluation, categorizing them into physicochemical, in vitro, and in vivo assessments, alongside specific techniques and examples of transdermal systems. The selection criteria for drugs and the importance of compatibility and stability in formulation are also emphasized.